The Mighty Catalyst
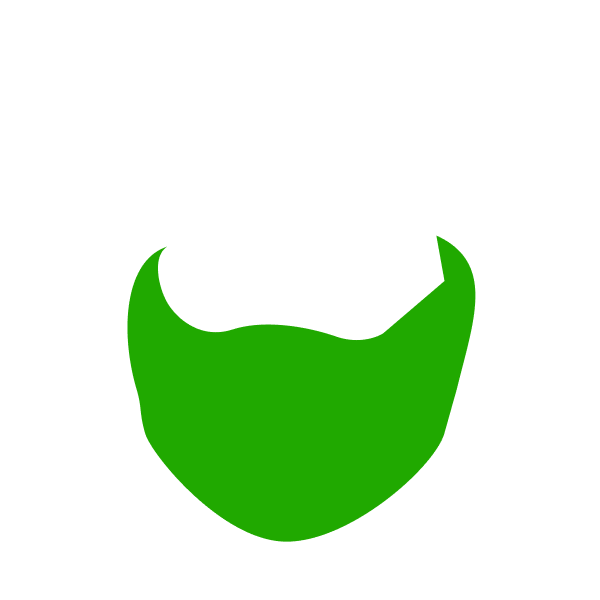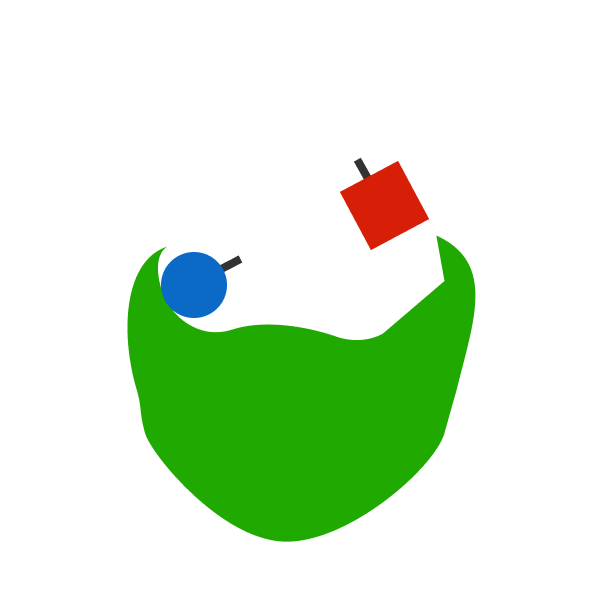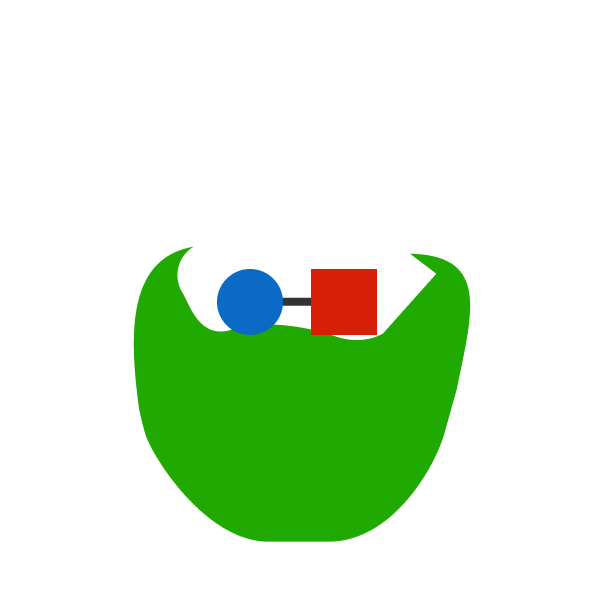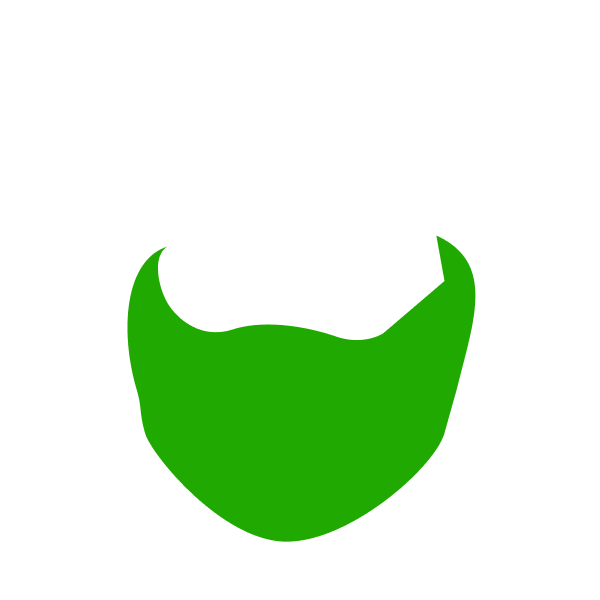
A catalyst is a molecule that can increase the rate of a chemical reaction.
Suppose we have a reaction that releases energy E . If we introduce a catalyst into the reaction and assume that no other conditions change, does the amount of energy released during any given reaction increase, decrease, or stay the same?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
7 solutions
I think that catalyst increases or decreases the activation energy of the reaction depending on whether it is a positive or negative catalyst... Here presumably we are talking of a positive catalyst the it will decrease the activation energy,so the energy released should not be the same... Please rectify me if I am wrong.
Due to the catalyst, the reaction goes faster and the energy produced per unit time increases. But the total energy produced in the reaction of the same amount of reactants under the exact same conditions remain always same.
In a simpler way: Energy produced per unit time (increases) X Time taken (decreases) = Same energy output!
It is unclear whether the question is asking for instantaneous energy or total energy (integral over the entirety of the reaction). Maybe even clarify that there is a finite amount of reactants.
Log in to reply
Another question that has more than one answer. assumption is the m of all fups
I agree. I took it to mean the amount of energy measurable immediately after introducing the catalyst, which would be greater than the measurement of a control group. Obviously the net at the end of the reaction would be equivalent for both groups.
It is definitely unclear whether the question is asking about a single reaction or over time. I "guessed" correctly because "increase" is also the immediate solution for someone who didn't really think about the problem, meaning that the problem maker would have avoided it.
"and assume that no other conditions change" - so time did not changed... Therefore the Energy increases right?
Log in to reply
Time is not a condition(i.e. Given at the beginning). Its a measurable factor of the reaction(i.e. Recorded on the observation of expected result).
The catalyst only lowers the activation energy which is the minimum energy needed to start the reaction, it has no effect on the amount of energy released.
The question is imprecise "does the amount of energy released increase, decrease, or stay the same?", if it's talking about the overall reaction, (and assuming the same equilibrium), yes, the energy is the same. But if the problem wanted to know the immediate rate, the amount of energy increases.
According to the problem, the catalyst increases the rate of reaction not lower the energy required for the reaction to occur.
Question unclear. It depends how you measure "the amount of energy released". Assuming it means total energy released until the reaction stops reacting, then yes it stays the same. But if it means total energy in any given amount of time before then, it would increase because more energy is released in that time.
A catalyst is a molecule which can increase the rate of the reaction without actually involving in it,that means it has nothing to do with the energy of the reaction.And a catalyst cannot change the free energy of reactants or products.
Catalysts have two mechanisms of action: They can either reduce the activation energy by stabilizing the transition state, or by providing a surface for the reaction to occur. The final product and the starting material are the same with or without the catalyst, and the energy released is dependent only on those two.
Theoretically, the correct answer is that the energy release stays the same. In reality, the energy release is often different. Chemical manufacturers often use catalysts not only to increase the rate of reaction, but also improve selectivity. That is, the catalyst increases the reaction rate of the high-value molecule which is desired while suppressing side reactions which create unwanted low-value molecules.
A catalyst can only affect the rate of a reaction. It has no effect on the energy released. For example, if that blue circle and red circle did not have the catalyst, it may have taken them a while to collide. But when they do, they will release energy E . With the catalyst, it takes less time for them to collide. But even so, they will still release energy E . The catalyst doesn't affect the reaction itself, so there is no reason for the energy released to change.