The Snake, the Dragon and the Ouroboros
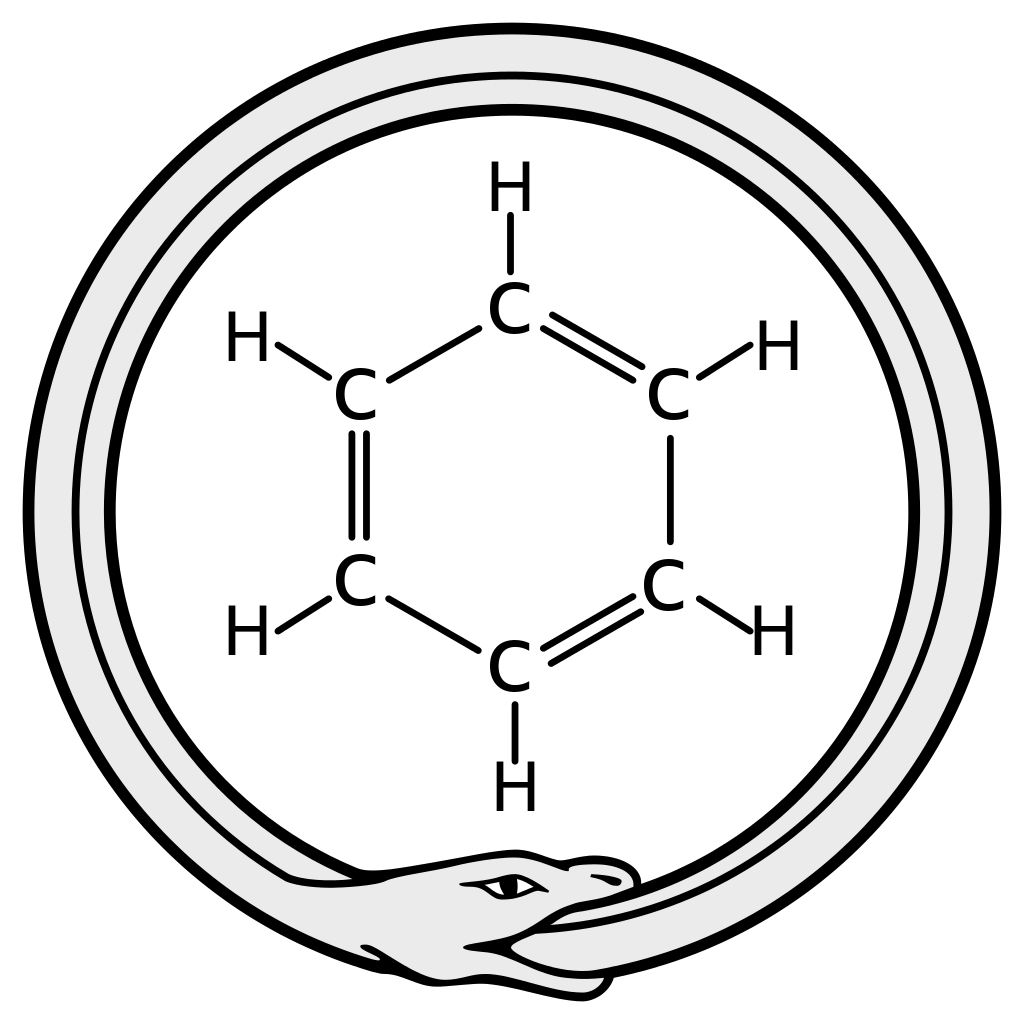
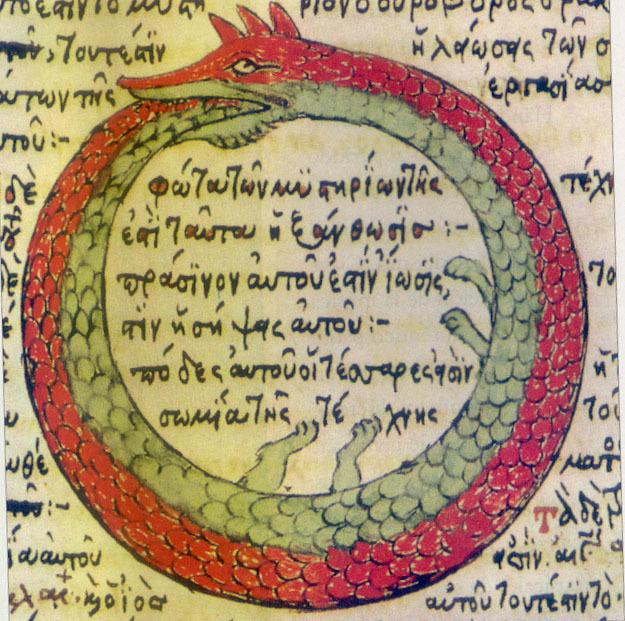
August Kekulé dreamt of the legendary Ouroboros to visualise the structure of benzene. However, benzene doesn't resemble the ouroboros that alchemy describes:
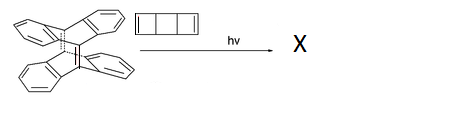
We know look into the synthesis of another compound that hasn't forgotten about chemistry's past. This compound was stumbled upon by researchers in the year 2003. The following scheme shows the reaction that they used. All other isomers are excluded as only one product, X , with all bonds in cis configuration is the one we are interested in:
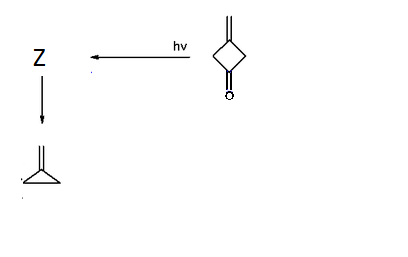
However not all conjugated compounds behave like an Ouroboros. One of these compounds has many interesting spectrochemical states. This compound, Z , is a short lived species that slowly converts into methylene cyclopropane and doesn't contain an oxygen atom :
The Aztecs believed that their god Quetzalcoatl , often portrayed as an Ouroboros , gave them cocoa from the plant Theobroma cacao . This plant is known to grow structures that resemble bird nests when infected with a fungi called Moniliophthora pernicosia .
Just like how Moniliophthora pernicosia exploits the gift of the dragon god, South Korean researchers in 2015 synthesised a compound extracted from this fungi by exploiting the non-Ouroborian compound that was previously discussed. This compound's structure is shown below:
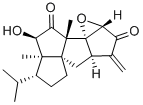
I is an important precursor that is obtained during their synthesis of the above compound. A key step is a [3+2] cyclo-addition. We define certain chemical compounds and systems:
- TMSCl denotes the Chlorotrimethylsilane group.
- TBAF denotes Tetra-n-butylammonium fluoride.
- TBDPSCl denotes the Chlorotert-Butyldiphenylsilyl group.
- Ts denotes the tosyl group.
- S_1 denotes the system of Oxalyl chloride, DMSO and a base.
- S_2 denotes the system of basic dimethyl (diazomethyl)phosphonate.
- S_3 denotes the system of tert-butyl hydroperoxide, titanium tetra(isopropoxide) and (R,R)-diethyl tartrate which was awarded the Nobel prize .
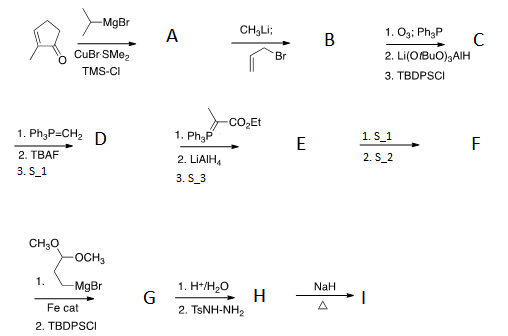
Using a mass spectrometer and an IR machine, the following are observed:
- D 's formula is
- E 's formula is
- F 's formula is and it contains a asymmetrical stretching vibration in the 2200 range.
- G experiences a asymmetrical stretching vibration in the 1900 range.
- I doesn't contain any nitrogen atoms.
If denotes the number of pi bonds in X , denotes the mass of Z , and denotes the number of rings in I , and the quantity is defined as .
Calculate the value of .
BONUS
- Provide a mechanism for the conversion of F to G .
- Is X aromatic according to Hückel's rule?
- How many spectrochemical states can Z exhibit?
- When is rotated by a ninety degrees through an axis perpendicular to the plane of paper what symbol is obtained?
All images belong to their respective owners.
The answer is 80.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!