The Sticky Water
Chemistry
Level
2
Angela is conducting an experiment. She pours water on three different surfaces. Which of the four would allow the adhesion force to overcome the cohesion force? - Glass? - Wax Paper? - or a Leaf?
Glass
Leaf
Wax paper
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The molecules of the water are attracted to the molecules on the glass. This doesn't occur on any of the other surfaces because the attracting force of water molecules to other water molecules are stronger than the attraction to the surface. An example of this would be when water sticks to a glass beaker, like in this image. Mercury has a very strong cohesion force, so it doesn't stick to the glass beaker.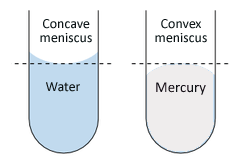
Important terms- Cohesion- When particles of the same substance stick together. (Polarity/ Hydrogen Bonds) Adhesion- When dissimilar particles stick together.