Thermal contact
Two identical bodies with different temperatures T 1 = 2 T 2 are brought into thermal contact, so that their temperatures are equalized and assume a final temperature T m . As a result of the temperature compensation, the entropy S = S 1 + S 2 of the overall system increases. What is the value of the entropy change of whole system, if this is given in units of the total heat capacity C = C 1 + C 2 ?
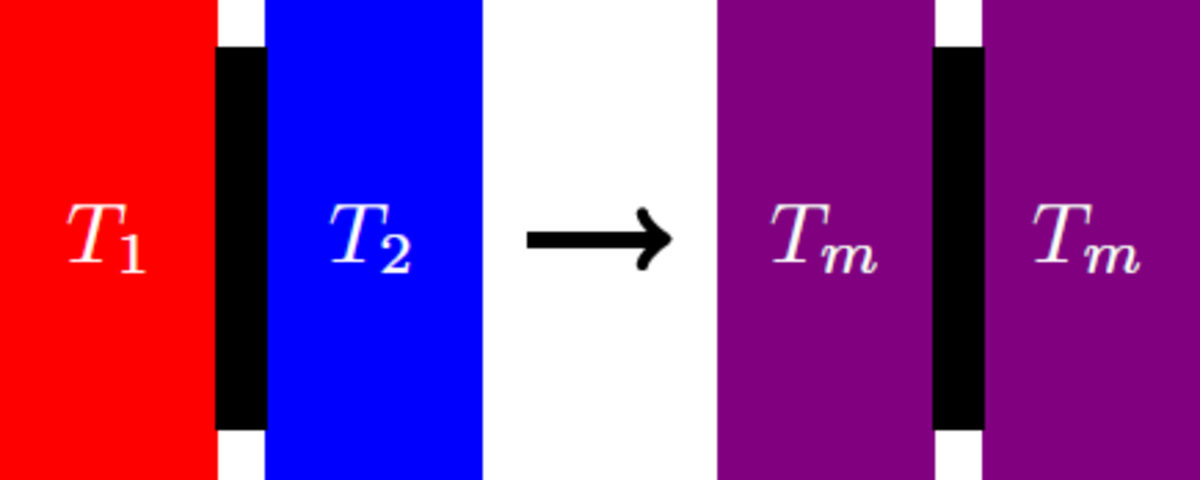
Details and Assumptions:
- T is an absolute temperature measured in units of Kelvin.
- The heat capacities of both systems are the same: C 1 = C 2 .
- No heat is released to the environment.
The answer is 0.05889.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
2 solutions
The final temperature T m results form energy conservation Δ Q 1 = C 1 ( T m − T 1 ) ⇒ T m = ! − Δ Q 2 = − C 2 ( T m − T 2 ) = C 1 + C 2 C 1 T 1 + C 2 T 2 = 2 T 1 + T 2 The entropy change is caused by a heat flow d Q i = C i d T i , i = 1 , 2 , so that d S i ⇒ Δ S i ⇒ Δ S = T i d Q i = T i C i d T i = ∫ T i T m T i C i d T i = C i ln T i T m = Δ S 1 + Δ S 2 = C 1 ln T 1 T m + C 2 ln T 2 T m = 2 C ln T 1 T 2 T m 2 = C ln T 1 T 2 T m = C ln 2 2 3 = 0 . 0 5 8 8 9 ⋅ C
The entropy of a system with constant (temperature independent) heat capacity is S = c ln ( T ) + c o n s t . (The constant term is not important here, but it is related to the fact the the heat capacity cannot be independent of the temperature, because according the the third law at T → 0 , S → 0 .) The entropy change is Δ S = c ln ( T f / T i ) , where T f is the final temperature and T i is the initial temperature.
According to the notations in the problem, c = C / 2 . The common temperature will be T f = ( 3 / 2 ) T . The entropy change of the first body is Δ S 1 = ( C / 2 ) ln T 3 / 2 T = ( C / 2 ) ( 0 . 4 0 5 5 ) . Similarly, for the second body Δ S 2 = ( C / 2 ) ln T 3 / 2 T = − ( C / 2 ) ( 0 . 2 8 7 7 ) . The total change is Δ S = Δ S 1 + Δ S 2 = C ( 0 . 1 1 7 8 / 2 ) = C ( 0 . 0 5 5 8 9 )