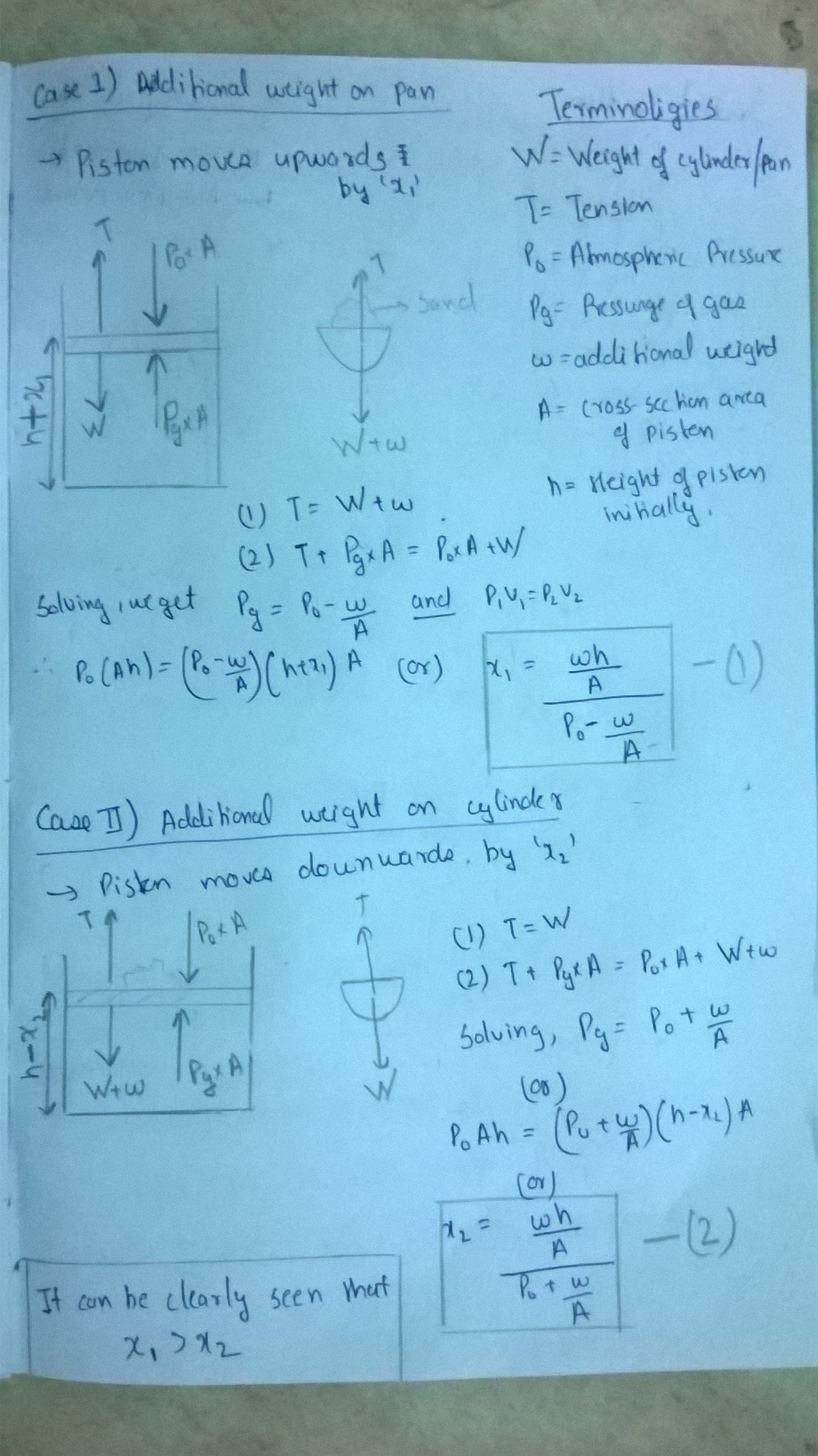
Thermo + Ideal Gas!
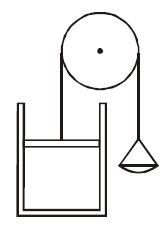 There is a sample of air at standard conditions in the vertical cylinder shown in the figure. The piston moves without friction and its mass is same as that of the pan on the other side of the pulley. Some fixed amount of sand is slowly poured either to the top of the piston or into the pan. The temperature remains the same. Then :
There is a sample of air at standard conditions in the vertical cylinder shown in the figure. The piston moves without friction and its mass is same as that of the pan on the other side of the pulley. Some fixed amount of sand is slowly poured either to the top of the piston or into the pan. The temperature remains the same. Then :
(A) Displacement of the piston will be greater if sand is poured in the pan
(B) Displacement of the piston will be greater if sand is poured on to the top of the piston.
(C) Displacement of the piston will be same in both cases
(D) Displacement of piston is greater or lesser in any case, will depend on the initial volume & pressure of the gas
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Solution 1: The easiest way to do this is to assume the additional weight is very large, then if the additional weight is kept on pan then the piston will move up by infinite distance as the opposing force is only force due to atmospheric pressure (constant). Whereas if the very large mass is kept on the cylinder, it moves down and the maximum distance it can move down is the distance of the piston from bottom (and the further it moves down , more compression of the gas results in a greater opposing force which will eventually balance the large additional weight).
Solution 2: For a mathematical answer: When there is no additional mass Pg = Po When the additional weight is added the system eventually comes in equilibrium.