Thermodynamically efficient or not?
A linear diatomic ideal gas (with adiabatic exponent, ) was initially at , and occupied a volume, . The gas is now taken through three thermodynamic processes as follows:
-
First: Gas is expanded isobarically to a volume of (Process AB) .
-
Second: It is taken through a polytropic process having equation with (Process BC) .
-
Third: After the polytropic process is carried out, the gas comes back to its initial temperature and an isothermal process is carried out in the third step and the gas returns back to its initial state (Process CA) .
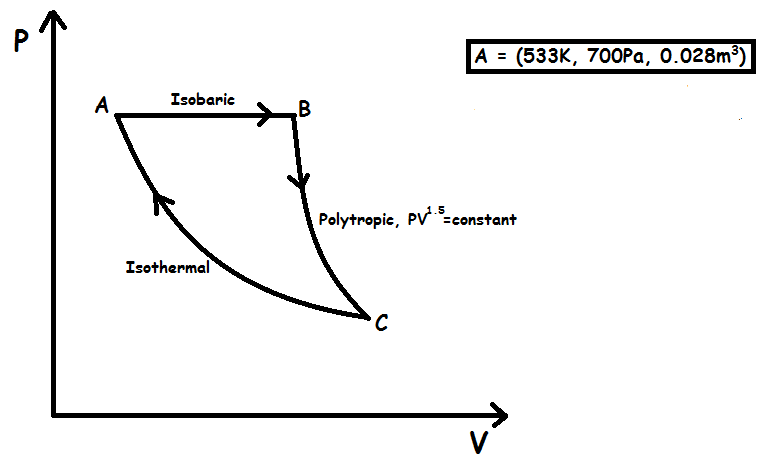
Find the percentage efficiency of the above cycle.
The answer is 38.63.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!