Thermodynamic+Chemistry
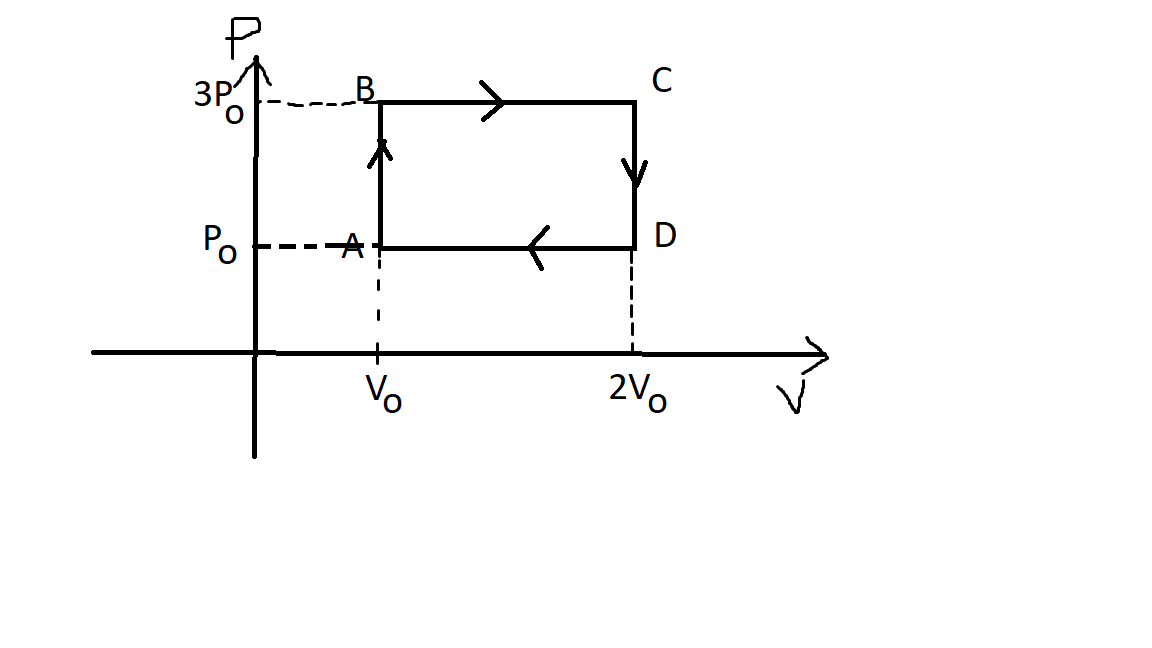
Consider the above cycle where the gas is diatomic.
Find the efficiency of the cycle ABCDA.( = η ) .
Now consider the following reaction(Unbalanced)
N X 2 + H X 2 → N H X 3
If the percentage yeild of the above reaction is 8 3 1 0 0 η .
At t = 0 , 2 moles of N X 2 and 8 . 9 moles of H X 2 .
If N moles of N H X 3 is obtained.
Then it is used in the following reaction to get product of [ A g ( N H X 3 ) X 2 ] X + .
The following reaction(unbalanced) was used for preparation of [ A g ( N H X 3 ) X 2 ] X + .
A g X + + N H X 3 → [ A g ( N H X 3 ) X 2 ] X + (Percentage yeild 100%) .
If the volume of container used in preparation of [ A g ( N H X 3 ) X 2 ] X + is 2 L .
Find concentration of [ A g ( N H X 3 ) X 2 ] X + in molarity after end of reaction.
The answer is 0.5.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Ratio of isobaric and isochoric specific heats of a diatomic gas is (7/5). The first law efficiency of the given cycle is (4/31). Thus the reaction yield of the first reaction is 50%. Therefore 2 moles of ammonia is produced in the reaction, and 1 mole of the ion is produced in the container. Since the volume of the container is 2 litres, the required molarity is (1/2) or 0.5