Top Bond
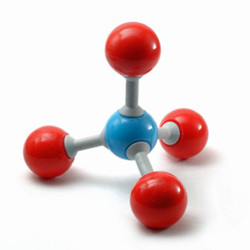 The ball-and-stick model of methane consists of 1 central carbon (C) atom (blue) and 4 bonding hydrogen (H) atoms (red), forming a tetrahedral structure as shown in the picture. All the 4 C-H bonds are equivalent and form an angle of about 109.5 degrees to one another.
The ball-and-stick model of methane consists of 1 central carbon (C) atom (blue) and 4 bonding hydrogen (H) atoms (red), forming a tetrahedral structure as shown in the picture. All the 4 C-H bonds are equivalent and form an angle of about 109.5 degrees to one another.
Approximately, how many times is the top H-atom higher than the C-atom from the floor?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
If we draw a line parallel to the floor along the C-atom, we can see that the top bond and the vertical axis are perpendicular to the floor and that parallel line.
The angle of any 2 bonds is about 109.5 degrees, so the angle that the lower H-atom makes with the floor is 1 0 9 . 5 ∘ − 9 0 ∘ = 1 9 . 5 ∘ from properties of angles of parallel lines.
Now, sin ( 1 9 . 5 ∘ ) ≈ 3 1 = Length of C-H bond Distance from C-atom to the floor .
In other words, the length of C-H bond is three times the height of C-atom. Thus, the height of top H-atom is (Length of C-H bond) + (Height of C-atom) = 4(Height of C-atom).
As a result, the top H-atom is about 4 times higher than the C-atom.