Two Radical?

Radicals play a key role in organic synthesis . Consider the following reaction occurs photo-chemically and proceeds through a stable bi-radical :
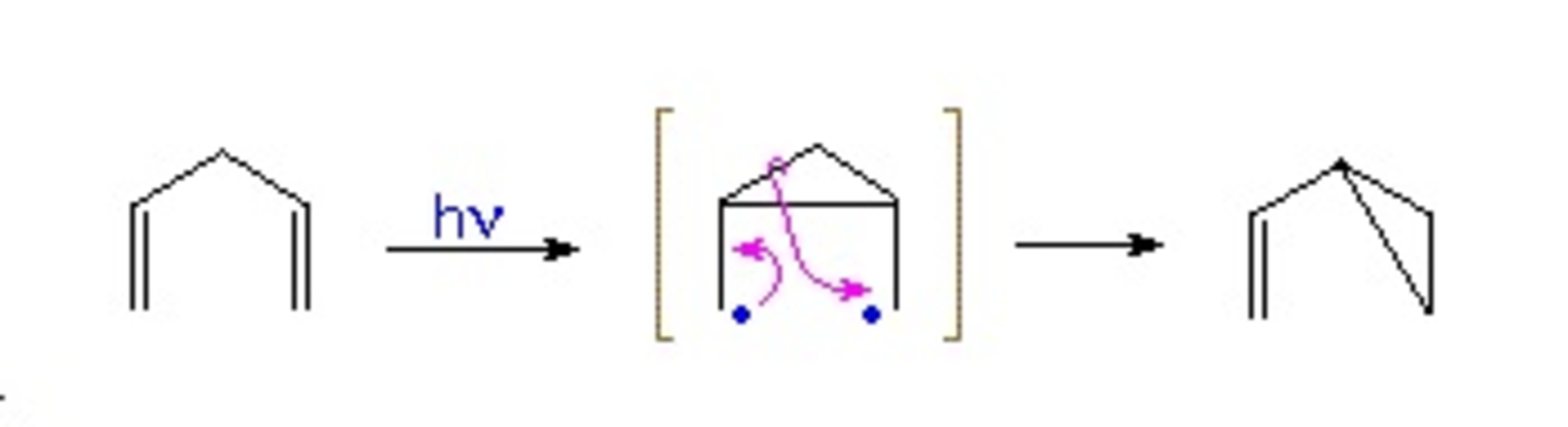
This reaction is used in the synthesis of a compound, isolated from Lycopodium magellanicum in 1976, by Chinese researchers. However, during a key step an interesting by-product formed through another mechanism :
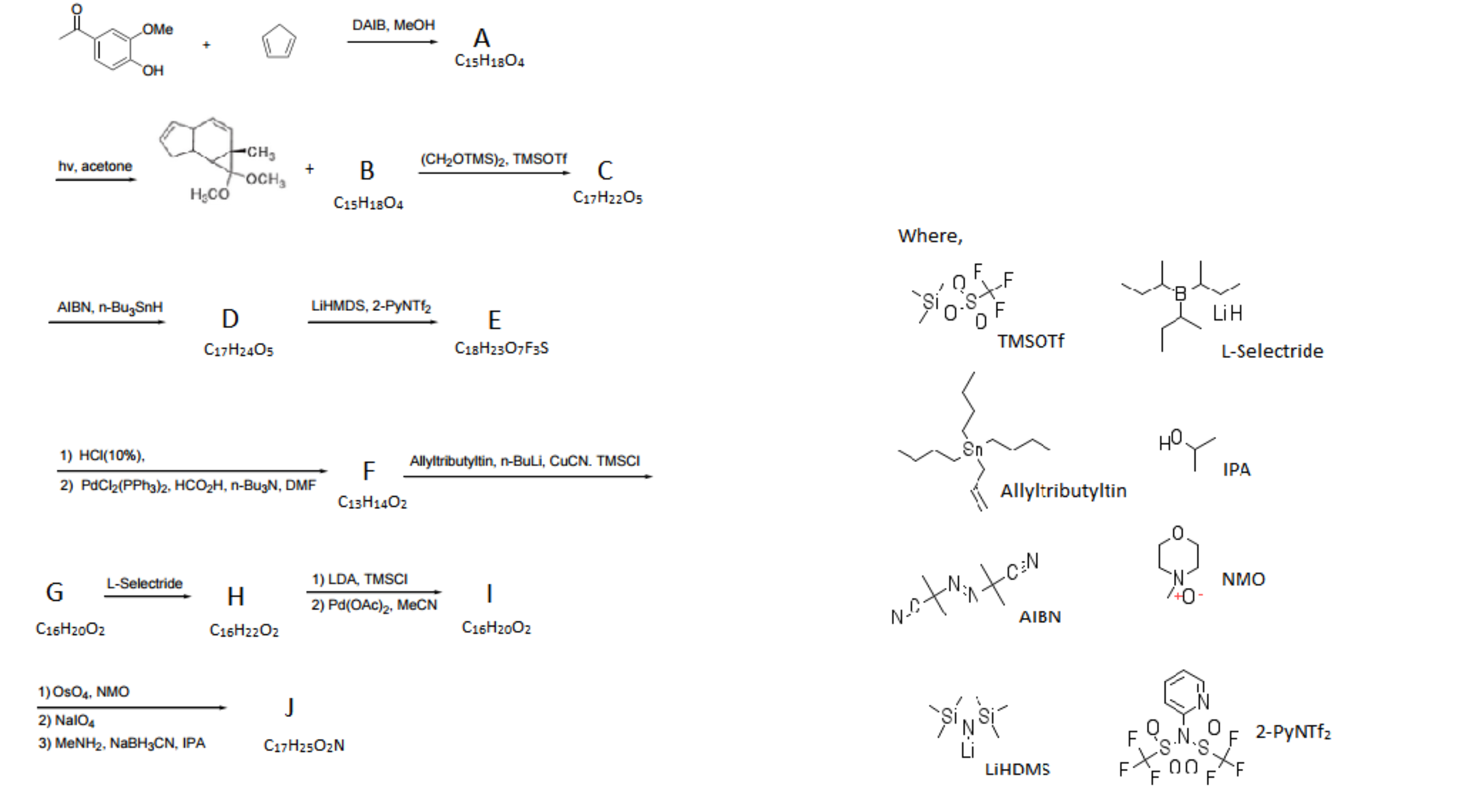
With a basic understanding of the mechanism involving the bi-radical, we now explore the reaction that gave rise to the by-product. Our exploration will look into another section of organic chemistry called skeleton synthesis. Through IR spectroscopy it is known to us that compound X contains no carbonyl group and Y contains no amide group.
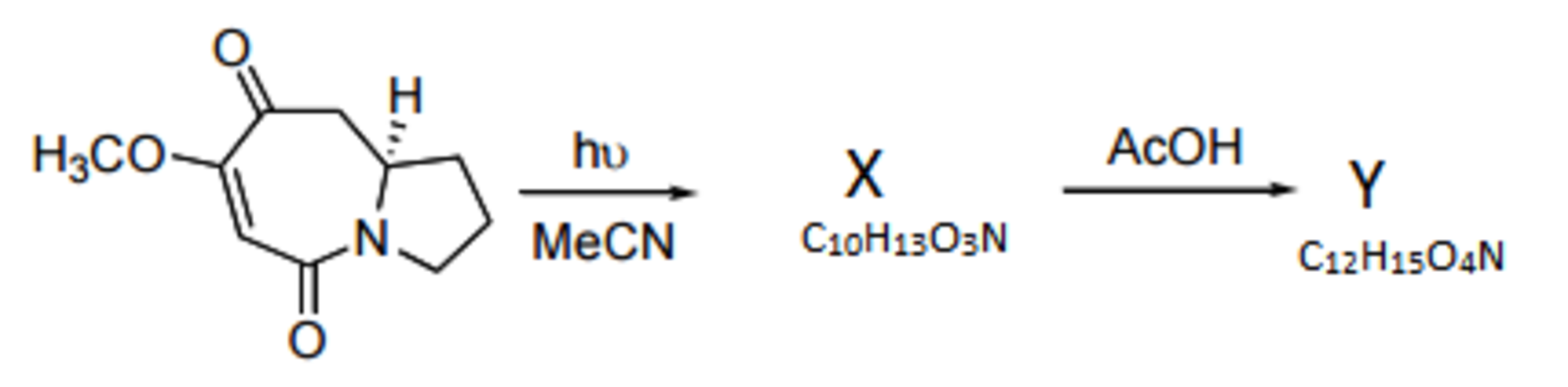
If denotes the number of rings in compound J and denotes the number of rings in Y .
Calculate
BONUS
- Provide an electron pushing mechanism for the conversion of A to B and X to Y .
- Deduce the structures of compounds A - J and X - Y .
All images belong to their respective owners.
The answer is 7.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
This problem does not require a prior understanding of all the reactions involved in the sequences.
The first reaction with cyclopentadiene should tell the reader that a diels alder reaction must occur. So we must start looking for a diene (note that a benzene ring cannot participate). A clue is provided in the structure of the by-product : an acetal . So the reagent DAIB and the solvent can induce a de-aromatisation through a tautomeric mechanism to form the required diene.
The structure of the cyclo-adduct is fairly simple to deduce and the next next should be obvious to the reader. The biradical mediated reaction must occur. Before moving on, we must make a key observation, which is illustrated below :
The numbers on the carbon atoms help understand how A will react to form B :
Now the structure of B is apparent to the reader. However let us redraw the compound for clarity, else we run the risk of creating a mess.
The next step is a variation of the popular ketone protection method which doesn't use an acid catalyst. This is done to avoid the decomposition of the acetal and provide regio-control with bulky TMS groups.
The following step is another use of A I B N ; reduction. It breaks open the cyclopropane ring by providing hydrogen radicals.
The next reaction is a Triflate group protected enolate that is formed by L i H D M S which is consistent with E 's chemical formula.
The next step is acidic hydrolysis. This implies that both acetals will decomposes to carbonyl groups. Knowing this we can compare the formulae to deduce that the second step is a coupling reaction resulting in the loss of the O T f group.
Allyl Tributyl Tin and the copper salt give Michael addition away and L-selectride's use can be deduced from the formula of H . The boron atom is bound to large secondary butyl groups, and hence steric hindrance helps in regio-control :
The next few reactions see the loss of two hydrogen atoms. The L D A and T M S C l reagent is to generate an enolate, one hydrogen down two to go. The second set of reagents' application is deduce by understanding that another hydrogen atom must be lost and that the T M S group must be removed. This implies a cyclo-condensation reaction must occur. This results in the formation of a six membered ring.
The set of reactions are simple and give the final product J that has four rings. Hence α = 4
We now move to the by-product. Observe that it looses two molecules of carbon monoxide this implies that a fragmentation reaction must occur. However the formula of X is the same as its precursor, hence this must be an example of a tethered mechanism. The hint provided states that no carbonyl group is present in X providing a clue to the reader. Consider the adjacent O M e group, if it were to fragment to give a methyl and enolic radical another carbonyl group would be formed which goes against IR data. Hence consider a hydrogen atom on the O M e group, fragmentation results in radicals that do not tautomerise. This hydrogen radical attackes the carbonyl oxygen to generate compound X :
Y is the product of acid catalysed lactonization and esterification made evident by the formula and IR data. The mechanism is left as an exercise for the reader. β = 3
α + β = 7