Ushering in a New Year
In hope of a less inflammatory year that mends the divide within communities across the globe we turn to the synthesis of a compound W that has a similar structure to the promising new anti-inflammatory drug Licofelone that possesses the following structure :
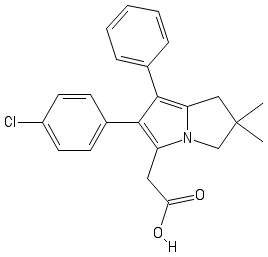
We start our synthesis using the methyl ester of proline . It undergoes the following set of reactions, a key step involves a Huisgen cycloaddition
- It reacts with Acetyl Chloride to give A .
- A reacts with a mixture of and to give B . It is known to us that B possesses the formula .
- B reacts with acetic anhydride on slight heating to form a compound C .
- C reacts with 2,2'-(1,2-Ethynediyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) to expel and gives us the product D whose formula was determined to be
- D reacts with 2 moles of in presence of Bis(triphenylphosphine)palladium(II) dichloride to give us the final compound W
We now define certain variables (all numbers are positive) :
- a is the number of rings in C
- b is the number of boron atoms in W
- c is the number of rings in B
- d is the difference between the number of signals in the NMR and the NMR of the compound W
What is the value of ?
Hint :
Compound C has no amide group
The answer is 2017.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
We start our deductions from the methyl ester of proline whose structure is as follows :
The formation of A is a simple acetylation reaction, by observing that A has a formula C 8 H 1 3 O 3 N , one can deduce that the reaction conditions favour a selective removal of the methyl group to generate a carboxylic acid B .
The next set of deductions start from compound D whose formula is known, the reaction conditions that lead to the formation of D favour a Huisgen cycloaddition (The alkyne and expulsion of C O 2 give this away), hence we find the formula of compound C to be C 7 H 9 O 2 N . This tells us that the transformation of compound B to C is a simple dehydration reaction that should result in a ketene . However, the hint provided tells us that no amide group is present in C implying that the ketene reacts to form a mesoionic bicyclic compound.
With most of the compound present, deducing the structure of W is a simple task. Knowing that W has a similar backbone as Licofelone , the large boron rings must be replaced with phenyl rings.
From the illustrations,
The answer is 2016 + 1 = 2 0 1 7
Inspirations :
Professor D.M. Volochnyuk's problem in the 4 9 t h IMO.