Vertical Flame Diversion

In normal conditions, when a flame is lit, it points upwards.
What would happen if we place the flame in a uniform electric field? We can create a uniform electric field using two parallel plates, and applying voltage across them.
A. The flame is attracted to the positive plate.
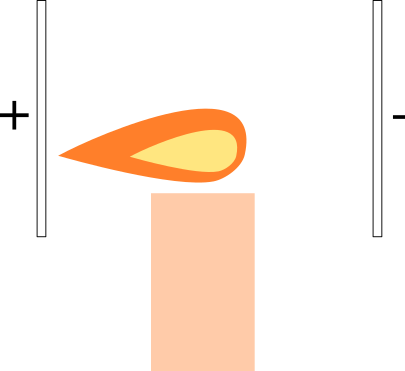
B. The flame is attracted to the negative plate.
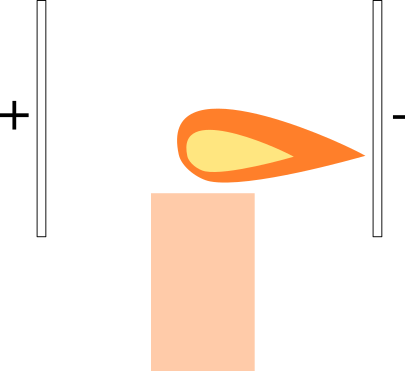
C. Nothing happens.

Note Assume an ordinary gas, wood, or candle flame
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
This really depends on the composition of ions in the flame plasma, and how the flame looks like depends on what's being burned. For the most part, ordinary gas flames have the bulk of ionic matter as positively charged ions, i.e., the negative ions are mostly electrons e − , while the positive ions are mostly the heavier reactants such as C O X 2 stripped of electrons. For such flames, the negative ions drift towards the positive plate, but the overwhelming mass portion of the flame drifts towards the negative plate, and this is the part that you "see" when radiant.
Not exactly a trivial question with a clear and unique answer. A typical ordinary flame is only barely a plasma, i.e., only a small percentage of the reactant molecules are actually ionized, but it's enough to pull the flame to one side.