Very Small Physics
The wavelength of radio waves is about while the wavelength of visible light is about As the wavelength of the waves gets smaller, their energy gets larger.
When we look into a microscope, our eyes are detecting light waves scattered from the object we're looking at, just as a radar tower detects radio waves scattered from planes. But you can't see individual atoms with a microscope. This is because, as a rule, a wave won't scatter off an object much smaller than its wavelength. It would be like trying to track a speck of dust in the sky with a radar station.
Particle physicists use scattering to study subatomic particles a million times smaller than atoms.
What can we conclude about these scattering experiments?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The correct answer is particle physics experiments must require very high energies (and small wavelengths) , for this reason, as we probe more deeply into subatomic particles, we'll need to build higher energy particle accelerators . Small objects will only scatter waves with very small wavelengths which must have high energies.
Light Microscopes
A light microscope is useful for discerning features down to about half the wavelength of the light used (usually around 5 0 0 n m light, with a photon energy of about 2 . 5 eV ). For visible light microscopes, this means that the smallest possible scatter sources (or objects) are about ≈ 2 5 0 n m . Biologists have come up with very clever ways to improve this limit, but it's unlikely that visible light microscopes will ever be feasible for observing features less than ~10 nanometers in size.
Photons
A single wavelength of light is composed of photons with a momentum related to their wavelength by the relation:
λ = p h = E h c
The smaller the wavelength of light, the higher the momentum (and the energy!). The electromagnetic spectrum extends from X-rays with very small wavelengths, to ultraviolet and visible light with intermediate wavelengths, to microwaves and radio waves with long wavelengths. The energy contained in a visible light photon is much less than an X-ray photon, and much more than a microwave or radio wave photon.
X-Rays
Since the energies of X-rays are so high ( E ≈ 1 0 k eV ), their wavelengths are very small ( λ ≈ 0 . 1 n m ). X-rays will scatter off of much smaller objects than visible light. For example, X-rays are commonly used for crystallography, where an intense beam of x-ray photons scatter off of every atom in a molecule. By collecting the scattered x-rays, we can take a snapshot of a protein and record the position of every scattering source. For x-rays, the scattering sources are single atoms, and X-ray crystallography can determine the position of every atom in complex molecules like proteins to extreme accuracy.
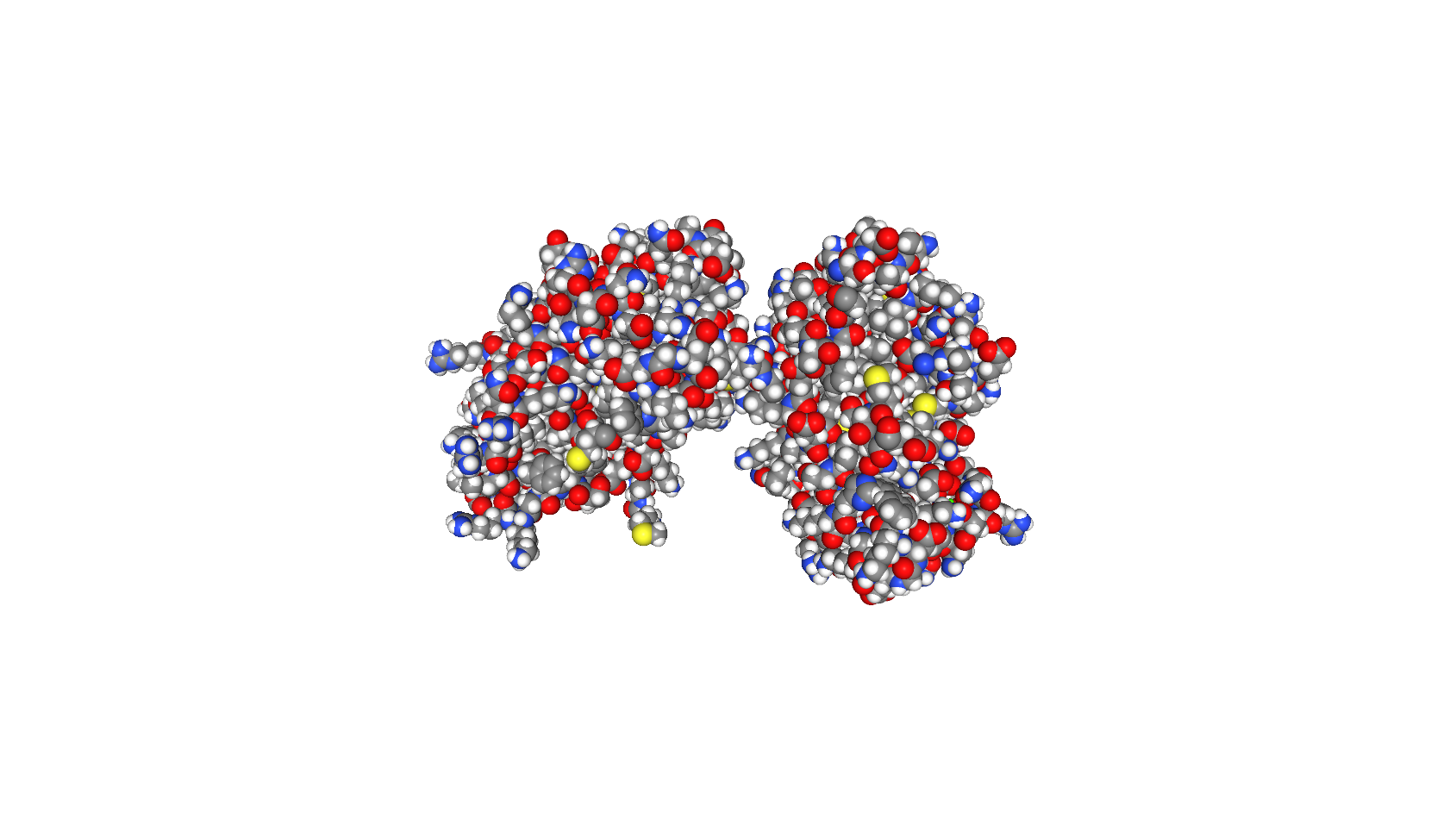 The crystal structure of a protein, each sphere represents a single atom
The crystal structure of a protein, each sphere represents a single atom
Electrons
But quantum physics tells us that light isn't the only thing that behaves like a particle with a wavelength. Ordinary matter like protons, neutrons, and electrons also have wavelengths. And since they are massive and have a high momentum/energy, they also tend to have very small wavelengths according to the same equation:
λ = p h = 2 m E h
Fast electrons can have very high energies ( E ≥ 1 0 0 k eV ) and tiny wavelengths ( λ ≤ 5 p m ). Electron microscopes can take images of molecules and track down the position of each atom: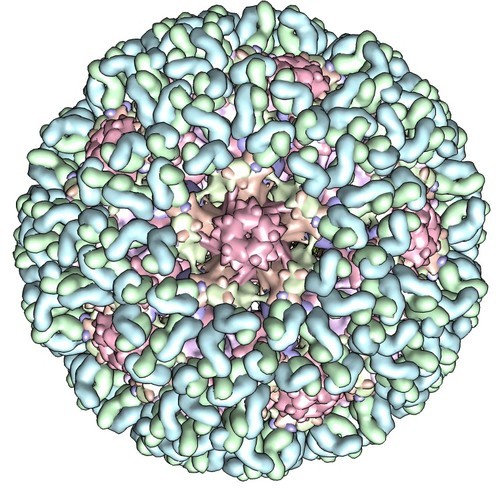 The structure of a virus, captured with an electron microscope
The structure of a virus, captured with an electron microscope
Particle beams
To probe systems even smaller than atoms and molecules, we need to make extremely high momentum particles that have correspondingly low wavelengths. This was first performed with beams of alpha particles , made up of two neutrons and two protons. Rutherford shot a beam of alpha particles at a thin piece of gold foil. Most of the alpha particles went straight through, but some scattered off something in the gold. The particles weren't scattering off the atoms, but off the nuclei , many thousands of times smaller than the radius of the atom, and this was the first high energy particle physics experiment to probe subatomic particles.
Rutherford's experiment showed that atoms were mostly empty space, with tiny scattering centers at the center of each atom. This could only be performed since alpha particles have such high energies, and small wavelengths ( E ≥ 1 M eV and λ ≤ 1 0 f m ).
Today, alpha particle beams can even be used as microscopes.
Particle Accelerators
To make extremely tiny wavelength and high energy particle beams, physicists accelerate particles (like electrons, protons, and even Lead ions) close to the speed of light. These accelerated ions can have E ≥ 1 T eV and wavelengths λ ≤ 1 × 1 0 − 1 8 m . Small enough to scatter off of quarks, the subatomic particles that make up protons and neutrons in the nuclei of atoms.
As physicists probe even deeper into the subatomic realm, they've had to build higher and higher energy (and lower and lower wavelength) experiments.