Water in the Manometer.
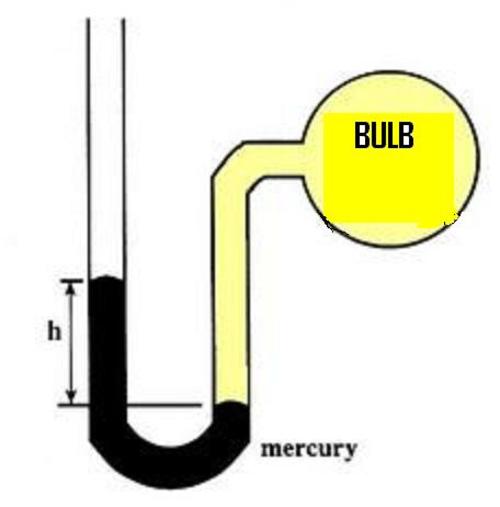 The system shown in the diagram is at equilibrium at
and the volume of the bulb is
which contains Oxygen gas and some amount of liquid water. If the bulb contains
of
, then the volume of liquid water is approximately
_
_
_
_
__
.
The system shown in the diagram is at equilibrium at
and the volume of the bulb is
which contains Oxygen gas and some amount of liquid water. If the bulb contains
of
, then the volume of liquid water is approximately
_
_
_
_
__
.
At Vapour pressure of water is .
.
The manometer is closed and the space above the other end is a Vacuum.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Vapour Pressure depends on a particular temperature. Pressure of the oxygen gas will be 161-28=133mm. Use PV=nRT for calculating volume of Oxygen gas and volume of liquid can be calculated by subtracting it with volume of container. Mind the units.