
The Rising Balloon's
Ram has two identical balloons filled with the same mass of helium. He heats one with a space heater and cools the other with ice, and then releases both balloons outside.
Which balloon will rise faster immediately after being released?
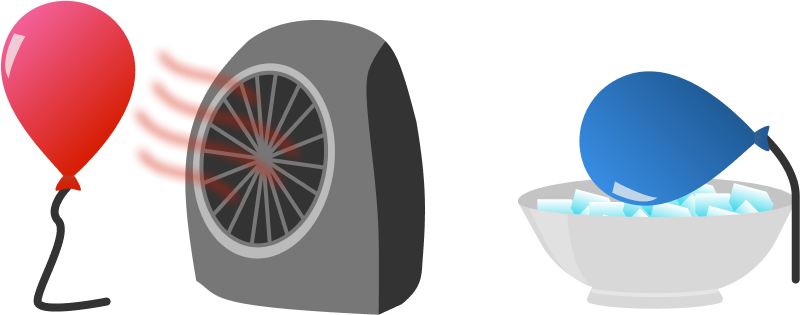 One balloon is heated while the other is cooled.
One balloon is heated while the other is cooled.
Assumptions:
- No wind is blowing.
- The balloons don't break when heated/cooled.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
22 solutions
Moderator note:
If you love science and need somewhere to learn the basics, try out our new course: Science Essentials
Science Essentials Showcase

Design Experiments
Learn about experimental design, to plan your own experiment!
Learn About Density
Discover how Archimedes was able to determine if King Hiero's crown was pure gold using density.
Explore Potential Energy
Determine the potential energy of a skydiver at the moment they jump!
Understand Temperature and Pressure
Learn about the relationship between temperature and pressure to predict when a balloon is more likely to pop.
Learn About Heat
Explore temperature, specific heat capacity, and heat transfer mechanisms.

 +2
+2

Great solution!
Or else, just "Warm air rises up" should help, right?
Log in to reply
Yes you are right but not makes a complete sense
The questions asks which is faster, not higher.
Log in to reply
The hot balloon experiences greater force on it, so its acceleration is more, so it rises faster too.
Well thought of question Ram. It had me stumped until I realized the solution!
This is my first question and I think I thought too much outside of the balloon. I am familiar with heat increasing molecular movement and volume, and how kind of how hot air balloons work. But hot air balloons are not sealed from the atmosphere, as a helium balloon. Does the hot air that escapes out of the bottom of the balloon utilize the convection currents for lift? Or is this operation more related to buoyancy?
Log in to reply
Upthrust also has contribution in rising the balloon. But the question says no wind is blowing so upthrust contribution is very little.
Can't the Helium molecules squeeze through the space between molecules of the balloon. In that case the balloon heated will have less volume of helium compared to the cold one at the same temperature??
Log in to reply
The question says that the balloon don't break while heating which means the gas cannot squeeze through the balloon..
Log in to reply
For the purposes of the question, I'm pretty sure the assumption is that the "pores" of the balloon do not allow for helium molecules to escape. I still struggle with the heat vs volume portion of this discussion. While the heated helium would expand due to that heat applied (after filling the balloon), it would seem that the lifting power of the helium would still be the same. But, the fact that AIR rises faster when heated, AND the fact that there is some amount of air in the balloon(s) would make for a good argument of the heated one rising faster... to me that is..
"balloon filled with hot air will have more volume than the balloon filled with cold air" Sorry, I thought they were both filled with helium.
Log in to reply
I wrote my solution in general to all gases that is the reason I wrote it as "air" .
Ok. For you convenience I have updated the solution. Hope that it is clear for beginners of the vast world of science.
what if the balloons are made of material that doesnt stretch, so they will have the same volume?? and they will rise at the same time??
Log in to reply
The lifting power of the same volume of helium which is then or cooled or heated has the same lifting power.
This thought experiment is flawed, as the gas inside the balloons would change only an imperceptible amount. I believe that there is the assumption that the helium inside has increased in temperature. However, the balloon is made from rubber which is a decent insulator (to a point) and therefore the gas inside the balloon shouldn't have increased in temperature as dramatically as first indicated. The same applies to the balloon cooled with ice. If the balloon contents had been heated or cooled, prior to being placed inside the balloons or changed in some manner similar to a hot air balloon after the balloons had been gassed up, then and only then would there have been a large enough change in gas temperature to modify the ascent between the two balloons.
Ideal gas law: PV=nRT
If T increases, what happens? n and R obviously don't change, so it must be P, V, or both. Regarding buoyant force, of P and V, it is volume that matters, because the buoyant force is equivalent to the weight of an equivalent amount of normal atmospheric air that would fill the volume. More volume = more air = more weight = more buoyant force. Balloon skin flexibility tends to allow stable increases in volume more easily than stable increases in pressure (this is why they are inflatable). A rigid container on the other hand, unlike a balloon, might tolerate increases in pressure but would better maintain volume. So higher temperature for a balloon should increase volume, therefore increase buoyant force, therefore increase upward acceleration all else being equal.
Assumptions not stated balloon is flexible and not adversely affected by heat or cold not necessarily break. Heated or cooled- it becomes rigid.
Isn’t this relative to the air outside the ballon? Hellium much less dense than air so heating or cooling relatively makes no difference?
P and V increase but same weight/mass and same air (n, number of molecules, is constant)
Hmmm from experience I said hot air rises, hence the hot balloon rises faster then the cold balloon.
Now this is my kind of answer!
The cold ballon has water drops on its surface. Hot air rises also
Log in to reply
Yes you are correct. Can you tell why water droplets form on both cold and hot air balloon.
Let's begin by characterising the property which makes the balloons rise.
This phenomenon is called
buoyancy
, which means fluid (air) exerts forces on objects (balloons) immersed in it.
The strength of this force is dependent on the volume of the fluid (air) the object (balloon) displaces.
Since both of the balloons have the same mass, effect of gravity onto them is the same,
meaning that the one which experiences higher
buoyant force
will rise faster.
Higher the volume, higher the
buoyant force
.
Charles's law
states that for gases
V
∝
T
when pressure is held constant,
where
V
and
T
denote the volume and (kelvin) temperature of the gas.
We can assume that pressure inside the balloons is pretty much constant,
because balloons are made of very flexible materials which would equalize the pressure by just expanding or contracting,
meaning that
Charles's law
holds for the gas inside the balloons.
This means that the balloon which has higher temperature will have higher volume
which will displace more air creating more buoyant force making the
Hot balloon
rise faster.
Like ships in water, the balloons in air experience an up thrust equal to the mass of fluid (air in this case) displaced. The warmer balloon has greater volume (air expands with increasing temperature and the balloon stretches) and therefore experiences a greater upthrust and rises faster.
Good. You answer is slightly different from others as you used the concept of upthrust.
We know that h o t air is lighter then c o l d air so the balloon that is heated will rise faster.
The density of hot fluid is always lesser than that of cold fluid,which is why baloon filled with hot air will soar higher compared to the other one filled with cool air.
the heated air moliculs will vibrate and air makes stuff simple
We know that change in temperature occurs due to enrgy change. If we put balloon in cold environment then it will lose some of it's energy and of we put it in hot environment then it will get some more energy which will accelerate the balloon more faster.
If you have ever seen a real balloon that carries people...you have seen they heat it up to ascend! the same principle applies here.
The heated balloon expands whereas the cooled balloon contracts. The gas in the heated (expanded) balloon is less dense than the cooled (contracted) balloon. Hence, the heated balloon will have greater eternal atmospheric pressure exerted on it, causing it to rise at a quicker rate towards the less dense atmosphere located at a higher altitude.
When you heat the helium in the balloon, it becomes less dense as compared to surrounding air. So, the balloon with hot helium tends to float on comparatively cold air because of its less density.
Can you prove it? Or at least give a scientific proof .Please give your solutions in full.
One variation of this question would be which balloon has a faster acceleration.
On hot ballon, volume becomes more than the cold due to thermal expansion of gas so more buoyant force act on it while weight s are same so net upward force on hot ballon is more than the cold ballon.
When molecules cool, they become denser, so the cold balloon will rise slower.
I answered based on my science teacher, when in a pot on the stove the warm water rises and the cold water sinks. This is a similar problem only with different objects.
But the only difference is that we are using helium instead of water.
helium will expand when heated and contracts when cooled, therefore the hot balloon will rise faster.
Conocemos de antemano que los globos con helio flotan porque su densidad es menor que la del aire, así que subirá más rápido el que tenga menor densidad, tenemos que densidad=masa/volumen, así que como las masas son iguales subirá más rápido el que tenga mayor volumen, el volumen se determina con las leyes de los gases que dice que a mayor temperatura mayor volumen, así que subirá más rápido el globo caliente.
Vro y'alls discussion complicated asf. Heat rises so the hot balloon finna rise faster
The kinetic theory of gases: 1/2mv^2=3/2kT
Now, in the case of the same gasses, m = const. Needless to say, k = const. If T increases, v increases. So higher temperature for a balloon should increase speed.
I have been struggling whether the drive force or the air resistance will increase more... which is beyond my knowledge, however , I find the air resistance is proportional to the πr^2 or less( because of its shape), while the motivate force is exactly proportional to (4/3) πr^3 ,for F(up)=V ρ(air) g-V ρ(in)*g=Vg[ρ(air)-ρ(in)] so, as the radius increases, the increase of air resistance is smaller than the increase of drive force by an order of magnitude.
we know that higher temperature increases the volume of the hotter balloon since the volume of gases is directly proportional to the temperature. Since the hotter balloon higher volume as compared to the colder balloon, it will experience more buoyant force by the air surrounding it.
FB = ρf *Vf *g where FB is the buoyant force, ρf is the density of the displaced fluid, Vf is the volume of the displaced fluid, and g is the acceleration due to gravity, 9.8 m/s2.
Also since they have the same mass they will experience the same downward force from the gravity. So the net upward force experienced by the hotter balloon is higher as compared to the colder balloon. Therefore the hotter ballon will rise higher.
We know that when temperature increases the space between the molecules (inter molecular space) increases . Also, when temperature increases the volume increases. So, the balloon filled with hot helium will have more volume than the balloon filled with cold helium. Further it is given that same mass of helium is filled so mass is constant .
Now, Density = V o l u m e M a s s
Now it is clear that when mass is constant, density decreases with increase in volume and vice-versa .
Therefore, the density of the balloon filled with hot helium is less than that of the balloon filled with cold helium. Hence the hot helium balloon will rise higher and faster.
Note : This is the theory behind hot air balloons.