Who has more?
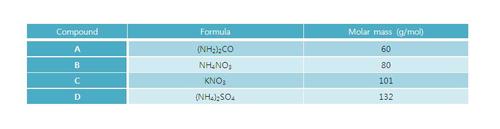 The above table shows some nitrogen compounds and their respective molar masses. Which of the following is the correct order for the numbers of nitrogen atoms per gram of each compound?
The above table shows some nitrogen compounds and their respective molar masses. Which of the following is the correct order for the numbers of nitrogen atoms per gram of each compound?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The order is A>B>D>C . See the table below.
Compound A B C D Formula ( N H X 2 ) X 2 C O N H X 4 N O X 3 K N O X 3 ( N H X 4 ) X 2 S O X 4 Molar mass (g/mol), m 6 0 8 0 1 0 1 1 3 2 # N atoms, n 2 2 1 2 n / m 0 . 0 3 3 3 0 . 0 2 5 0 0 . 0 0 9 9 0 . 0 1 5 2