Atmospheric physics 4: Ideal gas
Dry air behaves in good approximation as an ideal gas and therefore follows the general gas equation: \[ \boxed{P = R \dfrac{n}{V} T = R^\ast \rho T} \] where
- is the pressure
- is the amount of substance (in units of mols)
- is the volume
- is the mass density with the molar mass
- is the absolute temperature (in units of Kelvin)
- is general gas constant
- is the specific gas constant
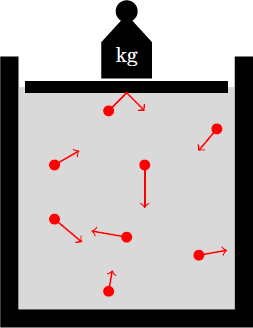
Ideal gases have a low particle density, so there is virtually no interaction between the molecules (except for rare collisions). Thus, the gas particles have random positions and velocities, that are completely uncorrelated. Therefore, the thermodynamic properties of the ideal gas can be derived from the kinetic theory of gases. Here we briefly outline the arguments that can be used to derive the general gas equation. The temperature of the gas is a measure of the kinetic energy of the individual particles. The pressure on the walls of the container arises because the molecules regularly hit the walls, each transmitting an impulse per second, which is proportional to its kinetic energy. The pressure generated by this collisions is thus proportional to the temperature and the (mass) density of the gas. Thus, the general gas equation results except for a constant pre-factor:
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
There are no comments in this discussion.