Charles' Law
The Ideal Gas Laws describe the relationship between pressure, volume and temperature in a fixed mass of gas.
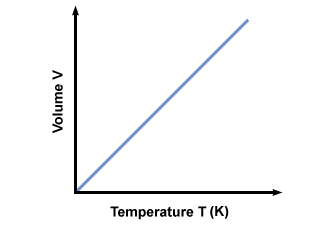
Charles' law describes the relationship between temperature and volume at a constant pressure.
The ability to visualize the behavior of individual gas particles in an enclosed space helps in understanding the mechanism underlying Charles’ Law. The molecules that make up a gas are moving in straight lines until they encounter another molecule, or a wall. When a molecule encounters a wall, it bounces off and moves off in a different direction. When this happens, Newton's Third Law of motion says that both the molecule and the wall will experience a force. In a flexible container such as a balloon, molecules hitting the inside of the of the balloon are what keep the balloon inflated. In a rigid, but adjustable container such as a sealed syringe, the collisions of the moving gas molecules with the syringe walls provide the force that resists efforts to move the syringe plunger.
Increasing the temperature of a volume of gas causes individual gas molecules to move faster. As the molecules move faster, they encounter the walls of the container more often and with more force. In an inflexible container, the more frequent and forceful collisions result in higher pressure. However, if the container volume is adjustable, the volume will increase, and the pressure will remain the same. Charles’ Law is the formal description of this relationship, allowing change in volume to be calculated if the temperature change is known.
The equation describing Charles’ Law is:
V1 / T1 = V2 / T2
assuming no change in pressure.
The relationship is linearly increase , if the temperature of a volume of gas doubles, the volume doubles. Temperature has to be measured in Kelvin, the absolute temperature scale, for the relationship to hold.
While Charles’ Law describes the behavior of ideal gases, not real gases, the law does have real world applications. Real gases behave in accordance with Charles' Law at temperatures well above the gases condensation point. Closer to the condensation point, the linear relationship does not hold up; volume decreases more rapidly than temperature.
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
Amazing! Very clear and well explained. ^^
Log in to reply
Thanks
Awesome!
Log in to reply
Thanks
17