Chemistry - Atoms
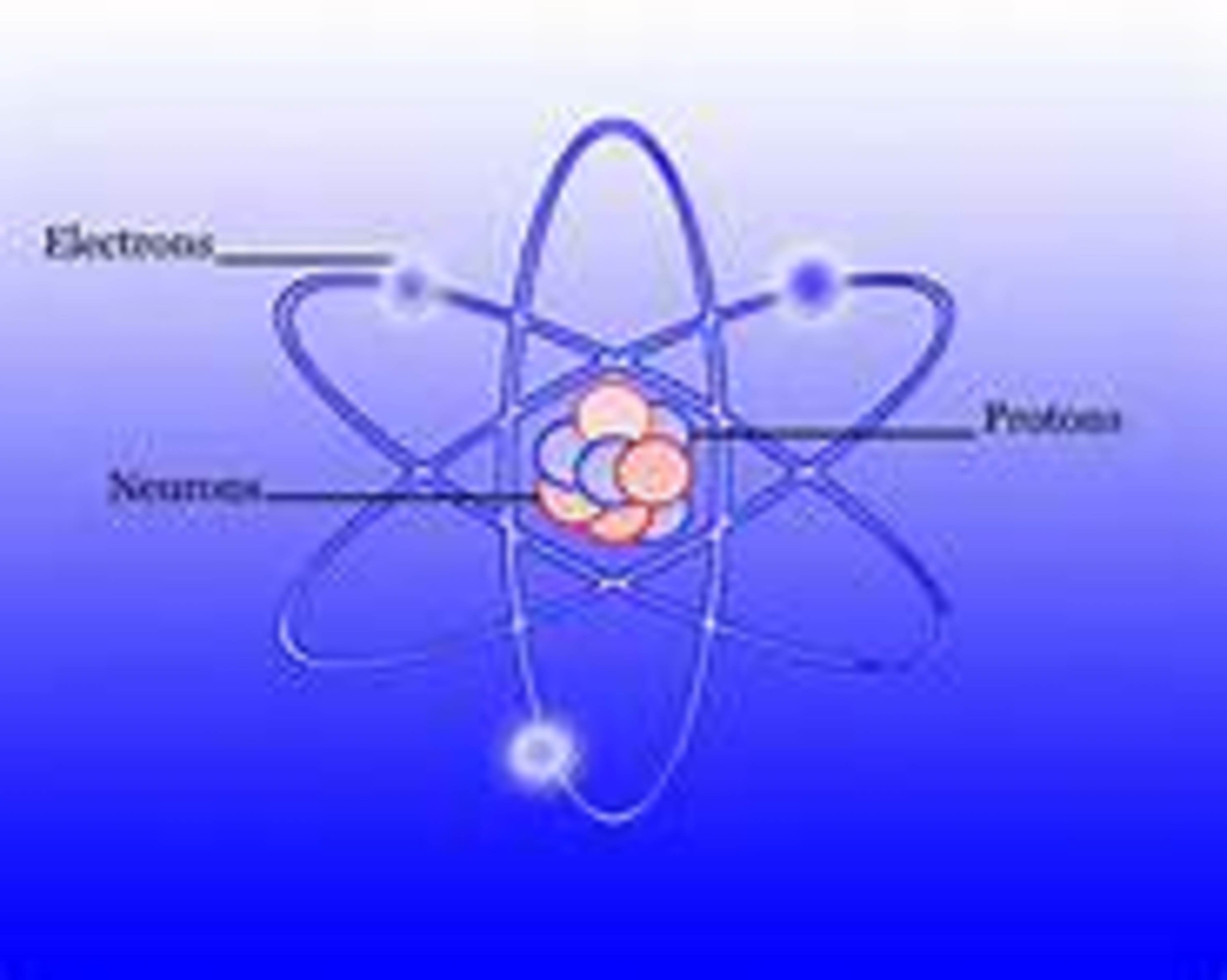
There are three basic parts of an atom. They are the proton, neutron and the electron
The proton has a positive charge, the neutron has a neutral charge, and the electron has a negative charge
Amount
Protons
- The number of protons is simply the atomic number of the chemical in the periodic table
Electrons
The number of electrons in an atom is the number of protons so that it can have a neutral charge
But an atom may also lose or get electrons and that may cause it to be an ion
Neutrons
You can get the number of neutrons in each atom here
Or, you get the mass number of a chemical, that is the atomic weight rounded to the nearest ones, then subtract it by the number of protons
In example,
the atomic weight of carbon is , so the mass number is
mass number = number of protons + number of neutrons
number of neutrons = mass number - number of protons
number of neutrons =
number of neutrons = in a carbon atom
Properties
Protons
- It is in the centre of the nucleus of an atom
Neutrons
- It is also in the centre of the nucleus with the proton
Electrons
The electron is special as it moves all around the nucleus because of the attraction of the protons. (It has a negative charge and proton has a positive charge, and they both will attract to each other like a magnet)
Its mass is about smaller than the proton
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
Do add quantum no.s too
i just han ana exam about it :D