Chemistry doubt
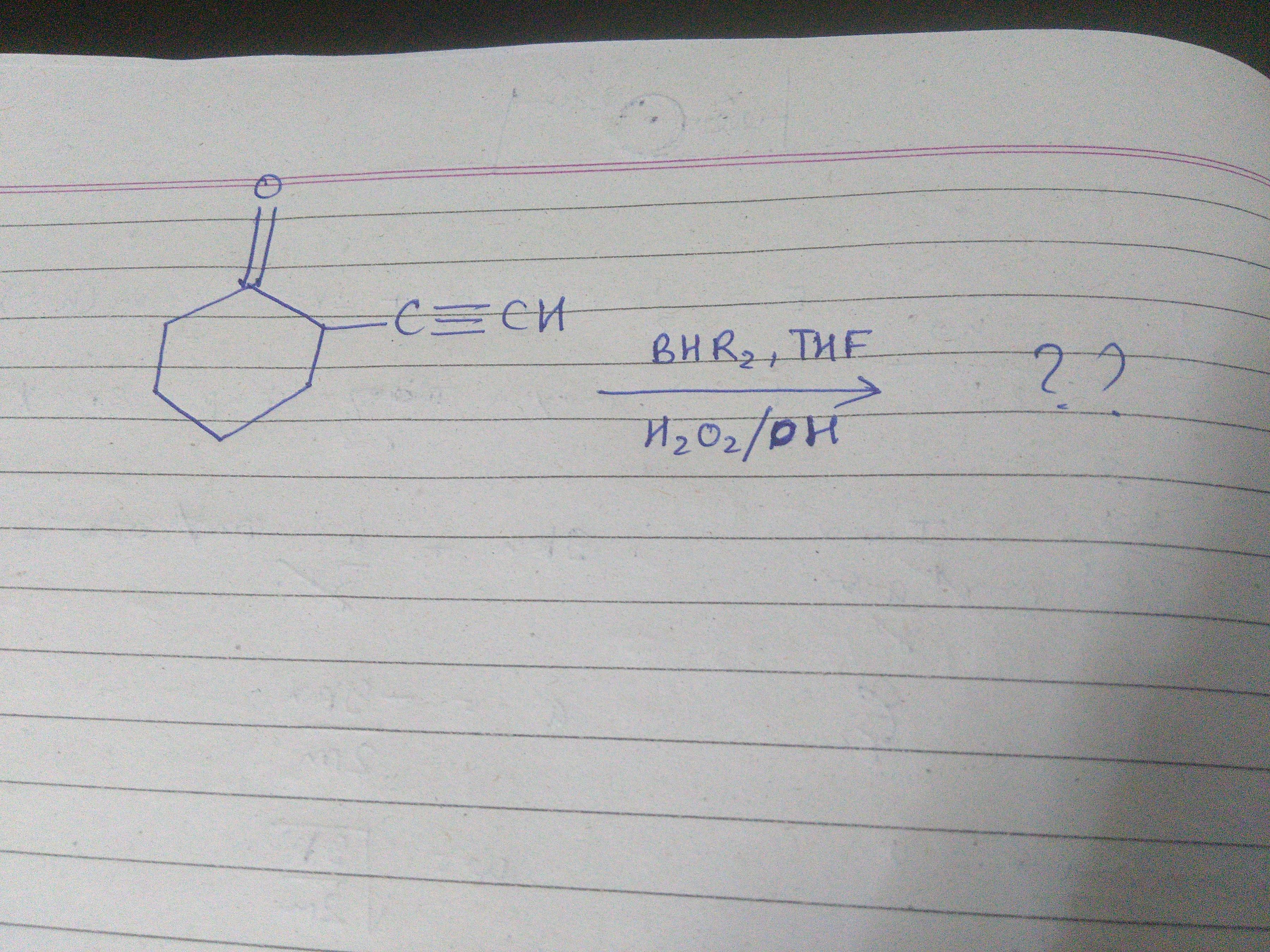
Well my doubt is that why will BHR2 attack the triple bond, instead it should attack the lone pair on the oxygen it is 'more' electron rich site that the triple bond?
No vote yet
1 vote
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
@Sumanth R Hegde @Prakhar Bindal @Spandan Senapati @Aniket Sanghi @shubham dhull
Log in to reply
Attack the triple bond and then tautomerise.
@Prakhar Bindal @Aniket Sanghi @Spandan Senapati Can anyone tell me whether the product be an aldehyde or ketone?
Log in to reply
Ketone...
BR2+ and H- attacks and as C+ will be more stable at last C (as in inner one C=O is there to destabilise ) so .... Rest u know...
Log in to reply
Is it due to c=o grp?
Ok thnx
carbonyl C=O Rarely acts as a nucleophile . moreover this site is prone to nucleophilic attack . Oxygen is sp2 hybridised and due to high s character its electronegativity increases and tendency to donate decreases
Log in to reply
Thanks a lot,
http://chemistry.stackexchange.com/questions/38006/carbonyl-oxygen-as-nucleophile
Log in to reply
for better understanding .
Log in to reply
Sorry for disturbing but one more doubt, if we attack an electrophile on phenol, will it attack on OH or the benzene? Bcoz when we react an electrophile with aniline, it attakcs nh2.
Log in to reply
I Cant understand exactly . can you tell which reaction of aniline are you talking?
Log in to reply
Nothing, I got it, thanks!
Protection in case of aniline.He is talking abt Nitration(EAS).
Ya because NH2 is better donating than oxygen and the reaction that you are talking abt is for protection in case of nitration..And a oxygen as such(keto one)is not usually taken as the centre for electrophilic attacks.
Log in to reply
OK . Well protection is done to decrease the mesomeric effect and in order to prevent getting ppt of tribromo derivative . Well that reaction has a pretty different mechanism called Nucleophilic Acyl substitution through tetrahedral intermediate . All carboxylic acid derivatives mostly undergo acyl substitutions (this reaction is pretty much importance with acetic anhydride)
Benzene definitely.And the same confusion may arise when we talk abt intramolecular reactions like ring formations.There even if you have such a keto group and other nucleophilic centres like OH,NH2.Attack takes on these centres.Because they can readily neutralise the positive charge by deprotonation.
N is very favourable in giving it's lone pair of electrons because of size and electronegativity factors but never in question solving try to add electrophile in stable O! C=O becomes reactive only in the case of nucleophilic addition to C=O which u will learn in 12th . Whenever you are to choose an better nucleophilic site then it's N compared to O!
In some cases we try up a mech by adding H+ on C=O without breaking the double bond like in esterification ... But that mech is quite exceptional and unstable .....
Remeber N acts as Lewis base as well acid! in numerous cases and this is very importamt fact to create q like of MgBr ones which are very reactive with H+
For q solving ... You asked above about the attack of it on O , guess our the product after that ... In exam if u get confused then apply logic of worthy product!
Log in to reply
Hmm so we don't have to bother about lone pairs present on carbonyl's oxygen during EAS.
Log in to reply
Especially C=O . Actually this confusion is arising coz you haven't studied whole of organic chemistry. once you study all of it you will yourself get feeling where attack should take place. Sometimes Phenoxide Ion (Anion of Carbolic Acid or Phenol) Can attack as a nucleophile , Sometimes With phenoxide ion O- does not attack and carbon of benzene attacks (in Kolbe Schmidt reaction) . So it depends on reaction conditions+attacking reagent and much more condition . as for now for EAS I Dont think you need to worry about it . you will study all these in April- May-June this year
Log in to reply
so @Harsh Shrivastava here also you can guess the product easily , phenoxide ion forms in basic medium and attacks Carbonyl compound ( it is nucleophilic addition reaction of phenoxide on carbonyls) so still the basic concept works, here phenoxide formed a normal bond , no utilisation of lone pair of O! It attacks on Carbonyl carbon! So here also no use of lone pair of O! Actually i don't seem to remember any non-exceptional rxn where O donated it's lone pair to form bonds. It can't entertain positive charge !
In that protecting group rxn ( Aniline + acid halide ( or anhydride) ) actually 1st N donates its electron to add nucleophilically on Carbonyl C of acid halide (on anhydride) and then H+ is released from N and Cl (or RCOO-) from the acid halide (or anhydride ) . This is not possible with phenol as O won't donate it's lone pair first! But ha one thing is possible that you add base which will take out H+ and then the so formed phenoxide ion adds as a nucleophilic on C=O (like prakhar said above )(eg rxn you can search in net) .
I hope so Harsh , now its clear to you about the lone pair of O! :)
Log in to reply
Hey so here we follow antimarkonikovs rule to add the H and OH and then Tautomerise.correct???
Log in to reply
In which reaction?
Log in to reply
This one only....
I Didn't get you . can you tell the substrate and reagent and where are you talking of tautomerism
Log in to reply
Hydrobaoration Oxidation reaction for a triple bond is associated with addition of H and OH(syn)following antimarkonikovs rule and then tautomerisation to the keto form.
Log in to reply
Acha HBO You are talking off . yep . its interesting to note R2BH Is used here unlike alkenes where B2H6 THF used
Thanks a ton! But the thing you said about protecting grps,... In the package of EAS, it is mentioned nh2 as.well as OH have electron density towards towards themselves, so OH should also interference right?
Bro @Aniket Sanghi and @Harsh Shrivastava . Phenolic Oxygen can ALSO DONATE its lone pair for acetylation . This method is extensively used for the preparation of Analgesic (Aspirin) . This reaction involves the reaction of Salicylic Acid with Acetic anhydride in H3PO4 or acidic medium .
Log in to reply
I know Bro! I didn't complete my statement that time as I were to go ...
Benefit It does donate lone pair only if it can immediately be relieved from the strain of positive charge @Harsh Shrivastava . That is it requires some supporting species that may help relieve it's stress.
Thanks! I don't know the names of reaction you are talking but we will study them soon our Fiitjee will start from 20 the March
Log in to reply
Our course is way ahead chem-Alcohols,phenols and ethers,Maths-Differntiability,continuity phy-Capacitors over....Courses in cm batches run really fast.
Log in to reply
We have nothing like cm batch here :( so there are only 4 batches.
Awesome speed we are wayy behind, our class 12 course is not yet started our classes will start from 20th wbu?
Log in to reply
And here we have around 10 Batches(2 CM).The first CM is for the top 30 and the nxt CM 2 for the next 40.Our classes have started and our course shall be over by July....Here when a major result is published it takes around 10-12 pages.Long list.
Log in to reply
Thats awesome our result is only 2-3 page long.BTW what are cm batches?
Log in to reply
Don't know although I have been in CM batch from 9th...Weird full form.forgot.☺☺
@Harsh Shrivastava,
The biggest hint here is THF, being a bulky hetero-cycle with an oxygen atom that complexes with BHR2 . The large complex will have a tendency to attack groups farther away from the cyclohexanone ring. Observe :
Consider substituent 1, it is 1 carbon single bond carbon and 1 carbon triple bond carbon away from the cyclohexanone which is approximately 154 pm and 120 pm respectively. This amounts for a collective 274 pm away from the cyclohexanone.
On the other hand, substituent 2 is only one carbon double bond oxygen away from the cyclohexanone ring which is approximately 123 pm.
With this understanding of the chemical environment, we now turn to the complex. When electrons are provided to the boron atom and complexing THF group must leave. This attack and leaving pattern mimics an SN2 or pericyclic pathway.
Hence, even though the carbonyl group contains a larger amount of electron density steric reasons stop it from reacting.
Log in to reply
So the product will be ketone according to your way also , right?
Log in to reply
I am not really sure about that... The BHR2 makes me think of bulky reagents like 9-Borabicyclo[3.3.1]nonane where steric factors predominate regardless of a pericyclic or SN2\ pathway. An aldehyde might form.
There are so many reasons why bhr2 will not attack carbonyl group! Are their any more examples like this where steric hindrance plays major role?
Log in to reply
Remember this, all reactions in organic chemistry are dictated by two things : electronic effects and steric hinderance.