Conversion practice 1
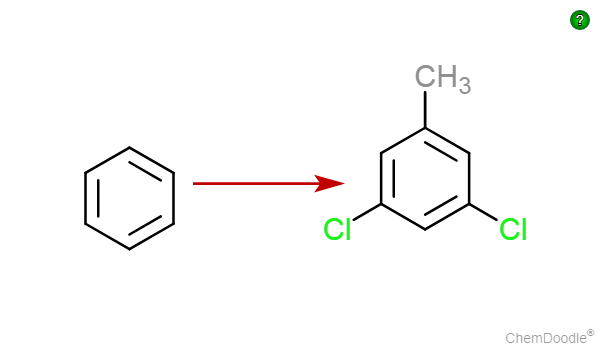
Just show the steps in the conversion! And you are done! And Remember.. This isnt a challenge... Or else It wouldnt have been so easy
Thanks
No vote yet
1 vote
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
@Tapas Mazumdar , @Sahil Silare , @Aaron Jerry Ninan , @Thomas Jacob , @Rahil Sehgal , @Sarthak Gupta , @Ayaen Shukla .
Please try this!! If you have good conversions, we can make a Chemistry Organic Conversion Contest.
Log in to reply
Hey!!!
Log in to reply
Hey!!! Whats up? You dont come to Whatsapp :(....
Log in to reply
Hmmm....its been many days
Log in to reply
I want some help in physics and all :P like always ...
Log in to reply
Log in to reply
Hmm, Nice way, Mynes was a bit longer :P. First added −COOH in the Ring. Then Nitration (2 eqv). Converted −NH2 to −NO2 and then to Diazonium Salt. Then CuCl ( 2eqv.) to make both of them Cl. Then added Red P, HI. Its all :P..
I know I am sillier than you. SO... silly method :(
Log in to reply
The method you've shown seems to work fine but I have some queries. 1. After the first nitration, the ring will be highly deactivated so the chances of a second nitration are very low. 2. How do you reduce -NO2 to -NH2 is also important. (Sn/Fe + HCl) works just fine, while reduction using hydrogen will reduce the carboxylic group as well. Rest of the reaction looks fine to me. :)
Log in to reply
Sn/Fe+HCl obviously :P ... Learnt from Aryl Halides.
Log in to reply
Great! I see you've excelled in chemistry. I remember the days you said your chemistry is the worst.
Log in to reply
No... Not excelled :P... Yeah.. Nowadays.. Chemistry seems interesting and scoring too.. Realised that, One can score in chemistry the most.... So to qualify exams... I study it and now.. It seems quite interesting..... But still P>M>C :P...
Log in to reply
Great! I've made chemistry my pillars of hope. C>M>P now :P
Log in to reply
Yeah.. Same.. its obviously the pillars hope..... :D... And after JEE when you come back to Whatsapp, Please share ur number :D
Log in to reply
Yes of course! :)
All the best for Advanced @Tapas Mazumdar!
Log in to reply
From me too @Tapas Mazumdar !!!! :D...
And thomas, Online aa :P
Log in to reply
Thanks. :D
Thanks. Hope to acheive better result than mains. :)
Log in to reply
Oh btw, Whats ur rank in JEEM?
Log in to reply
7899 :/
Log in to reply
Ohkay. Not that Bad. You are getting NIT. Now prepare for JEE Adv. to the fullest! And dont take much pressure... You were a Bio student. You managed Bio also in 12th.... So Your rank is quite nice!! :D
Log in to reply
Bio student. Haha :P No I was purely PCM student. And yes would prepare my best now. Thanks! :D
Log in to reply
But didnt you had Bio in 12th?
This method does not seem correct to me because when you do chlorination once on the aromatic ring then chlorine attributes (even though weakly) to activate the aromatic ring. The next chlorination will then happen at ortho or para position with respect to the first chlorine added and not at the meta site of the -CHO group.
Log in to reply
Yeah... But why Isnt my correct?
Oh yeah .....thanks @Tapas Mazumdar
Log in to reply
You're welcome. Btw you're also a very talented individual I must say. Both of you. You've practiced physics way better than I have. I still get baffled by some of your problems @Aaron Jerry Ninan
Hi!
Log in to reply
TOP!!!
Log in to reply
True that!!!!
Log in to reply
You too!
You TOP!!
Log in to reply
Abe Top chor... :P... Hangout pe aa..
Log in to reply
Ha
@Aaron Jerry Ninan , Hey!! try this once... https://brilliant.org/problems/lets-start-preparing-hard-for-kvpy-sx1/?ref_id=1491122