Coordination Compounds
I had an acquaintance with this question of coordination compounds which I find difficult to solve:
We have a coordination compound of ML type structure where the ligand L is
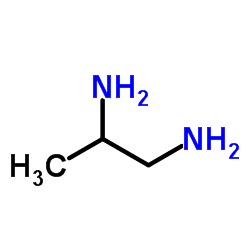 .
.
Now find the total number of stereoisomers.
Please help me through this problem!
No vote yet
1 vote
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
The ligand L stated above is a methyl substituted derivative of ethylenediamine ( NHX2−CHX2−CHX2−NHX2 or en ). This ligand is a bidentate chelating ligand where both the nitrogen atoms donate their electrons to the metal ion. Thus the ligand has a coordination number 6 and not 3 and it looks like a propeller, like this example
As you can see this has three chiral centers and no restricted rotation sites. So by using the three formulas of finding the number of stereoisomers when number of chiral centers is odd as listed below
No. of enantiomers =2n−1−2(n−1)/2No. of meso compounds =2(n−1)/2No. of optical stereoisomers =2n−1
We respectively get
No. of enantiomers =2No. of meso compounds =2No. of optical stereoisomers =4
Log in to reply
That's true but I faced problem in counting the cases of geometrical isomers in each case above.
Log in to reply
Is my answer correct? Do you know what the answer is? I didn't make a count btw, just went with the formula.
Log in to reply
The answer is 24 stereoisomers. Your answer must be correct regarding optical isomers as I had tried it (in another way) and got the same number of optical isomers but I am not certain.
Log in to reply
Yes, I got the answer. The number of optical isomers is 4 along with that if you also account the position of methyl group on each ligand, you can have a possible of 6 configurations wrt position of methyl group, thus 4×6=24. But this is the total number of all possible isomers.
Log in to reply
I will think on this and get back.