General doubt in isomerism
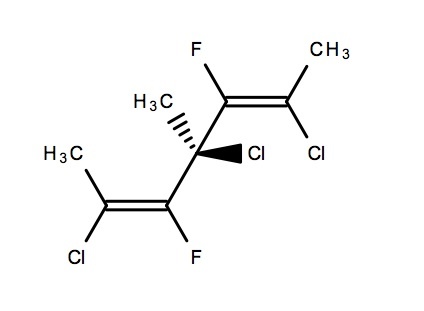
My doubt is is the compound above chiral or not?
Please can anyone tell in conformation?
No vote yet
1 vote

My doubt is is the compound above chiral or not?
Please can anyone tell in conformation?
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
In my opinion it's chiral, if one side you take E isomer and the other side the Z isomer the it would be an enantiomer and hence optically active
Hey @neelesh vij This compound is chiral because its mirror image is non-superimposable.
Chiral as it has only one chiral centre.
Log in to reply
The question is - Is that a chiral Centre!
I have already given the answer though.
What are your observations here?
Log in to reply
the points to favor is that both the two groups are same but they are cis and trans so the compound can be chiral. but i am not sure that's why i asked this.
Log in to reply
It is chiral carbon Because the left and right sides when are opposite (cis and trans ) the make the poparised light to rotate
Log in to reply
but the cis and trans carbons will rotate the light equally in opposite directions (as all the groups are same) so there will be no effect due to them. my main point is that whether the middle carbon will be chiral or not.
Log in to reply
How are you saying the cis and trans groups will rotate light in equal and opposite directions? I agree with @Gauri shankar Mishra, the compound is chiral.
Log in to reply
Umm. i am not sure about the statement. I thought it similar to R and S configuration. so does that mean geometrical isomers do not rotate light?
See buddy that lack of symmetry makes a compound chiral .and also tell me the question of himanshu and ms in which u have problem
Log in to reply
Try out ms chauhan ch: isomerism qno: 65,113,161
himanshu pandey qno: (level 2) 22
Log in to reply
I too have doubt in himanshu Rest are done and can be explained by me( ms questions)
I Think that This Compound Is Chiral . The central chiral atom is called pseudo chiral as the groups attached to the carbon can be different due to stereoisomerism.
Log in to reply
Me too.......same explanation...I am with you
But I dont see any chiral carbon in the structure.. Do you? ANd if thats true than the compound should be achiral...
Log in to reply
The central carbon, the one connected to the two double bonds has 4 different groups.
Log in to reply
No only 3 are different- methhyl,chlorine and other two are same- I guess..
Log in to reply
One is the trans compound, the other is the cis one.
Log in to reply
Oh Now I see... Thanks.. :)
I guess it is not but I am not sure
This is chiral. There is a central carbon bonding with 4 different compounds.
Ciral