Lectures on Quantum Mechanics 2: The Schrödinger Equation
From Wave to Particle
Louis de Broglie taught us that all particles in the universe possess a wavelength. We will consider microscopic objects because they closely follow the wave-particle duality more than macroscopic objects. Consider a particle travelling in space with no potential holding it down: we will call this the free particle. Since microscopic particles also behave as a wave, we will model the free particle as a plane wave enveloped in a corresponding wave-packet. The plane wave propagates in space and evolves in time: with a corresponding wave-packet:
 Wave packet
Wave packet
Using de Broglie’s equations of momentum and energy we can rewrite the wave-packet equation as
What can we do with this integral so that we can describe a physical system? Why not take derivatives until two sides of an equation match? Let’s begin by taking the time derivative and two derivatives in space:
Now there is one final step to do: balancing. If we multiply the time derivative by and the second spatial derivative by , we get
(this can be easily verified using the de Broglie equations).
It is customary in physics to describe physical systems in terms of total energy, which is the sum of the kinetic and potential energies. So all we have to do to this free particle is add to the right hand side. Hence,
Operators
Quantum mechanics is inherently linear, which means linear algebra (more specifically linear operator algebra) is the language of QM. Thus, it is most appropriate to write the Schrödinger equation in operator form.
An operator is an instruction that is acted on a function. An operator is linear if it satisfies the identity where are scalars. This is where QM starts to get abstract, so I will introduce some concrete examples. Consider the operators and If we act on some position quantity , we get the differential Hence, momentum is a differential operator. Looks straightforward enough! But consider what we can do to operators
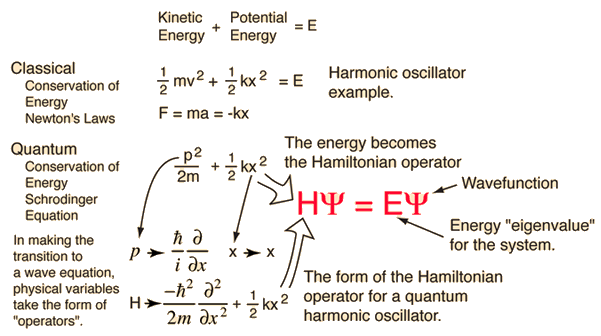
which is convenient. Now let us look at the Schrödinger equation again…in operator form
For the time-independent case , we replace the time derivative term with total energy, and if we want to be even more compact, we may denote the operator on the right hand side with the Hamiltonian operator .
Notice how this is shorter? Well, that's good and all, but not the point. The point is that we are acting a Hamiltonian operator on the wavefunction , which is convenient for reasons unclear at the moment. You see, linear operators can describe many things, but all linear operators follow the same algebra. We have entered the realm where abstract algebra infiltrates all of physics for the convenience of problem solving. So from now on, we consider operators to act on physical quantities and objects.
Determinate States
When you solve the time-independent Schrödinger equation, you will encounter values of energy that satisfy
These are called determinate states, which are eigenfunctions (also called eigenstates) of the linear operator with corresponding energy eigenvalues . When a linear operator possess several eigenvalues, we call the set of eigenvalues its spectrum. If two or more linearly independent eigenfunctions share the same eigenvalue, we call those spectra degenerate. You will later learn that degeneracies play an important role in describing the atom; hence much of quantum chemistry and solid-state physics. But for starters, you should solve the 1D particle in a box (Problem 3) below and calculate the corresponding energy eigenvalues .

Visit my set Lectures on Quantum Mechanics for more notes.
Problems
Use the de Broglie relations to obtain the dispersion relation of wave-packets. Hint:
1D Particle in a Box Consider the potential that is 0 within the region and infinite elsewhere. Solve the time-independent Schrödinger equation for this potential with boundary conditions: . Once you find the wavefunction (solution), normalize the wavefunction .
Note: is just
Now that you found the solution to the 1D Particle in a Box, find the set permissible eigenenergies (energy levels). Remember that the wavenumber of a matter-wave is given by where . This will help you simplify .
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
Nice presentation. It'd be great if you could show how Schrodinger's equation is the same as the diffusion equation in classical mechanics, but in complex time. This is the reason why Richard Feynman thought that quantum behavior could be modeled as a random walk in complex space. Too bad more isn't being said about this.
Log in to reply
I think the reason why more hasn't been said on Feynman's idea is that the probabilistic interpretation of QM showed infallible accuracy. The diffusion equation still relates in the probabilistic interpretation, since the wave-packet of observed quantities disperse if you stop observing for a while.
Log in to reply
What Feynman actually said about attempting to model it as a random walk in complex space, you first need a quantum computer---and back in those days, "quantum computers" was just a wildly speculative idea. His point was that you can't duplicate quantum behavior using classical means. But we need to distinguish "duplicate quantum behavior" from making predictions as per equations of quantum mechanics, the latter quite doable with conventional computers.
Is there any pdf or a source or website where these algebra are explained from scratch, i am familiar with grad, div, curl and greens and stokes theorem and their elementary applications in finding line and surface integrals, so anything to build upon that ?
thank you,
Log in to reply
div,grad, curl, laplacians are differential operators but operator algebra is much more general and diverse. There are too many operator algebras. IN QM, we focus on linear operator algebra.
@Steven Zheng Nice lectures Could you please upload a few more problems on QM.