Mechanism Challenge - 1

I wanted to make a monthly segment (if possible) presenting a challenge to the chemistry community here on Brilliant. This will be the first (of hopefully many) challenge(s):
Furan is an aromatic heterocylic that is known to participate in pericyclic reactions. The most common way of preparing such a ring is through metal mediated cyclization reactions.
The following conversion has no additional reagents and the initial compound is mildly heated to obtain the product. The conversion involves three steps:
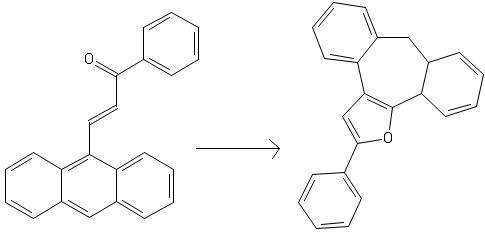
A mechanism can be either hand drawn or made using an online web tool. An answer will be provided in 96 hours (or earlier) if no one can provide a suitable mechanism.
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
Invitations @Prakhar Bindal @aryan goyat @Archit Agrawal @David Hontz @Dylan McQuiston @puneet mangla @Teleanu Florin and all Chem lovers .... I am already invited ! :) Right? @Vitthal Yellambalse
Log in to reply
u forgot me :( bro ???
Log in to reply
Oops sorry ! Actually I surfed the Chemistry column of community and invited those who have posted hard level problems (due to lack of time ) .
Log in to reply
now i have posted one ,plz do try bro-->aniket sanghi :) https://brilliant.org/problems/chemistry-for-life/#
I am also lover of chemistry
Yes you are. Don't forget about the correction!
ANSWER
The given figure hides some details about the reactant. It appears as though one large conjugated system exists but in reality, because of steric hindrance, the ring system tilts out of the plane. This splits the compound into two smaller conjugated systems. since we are just heating the compound, all reactions must be pericyclic in nature.
We start by noticing that within this large compound a diene (circled in black) and a dienophile (circled in red) can form a Diels-Alder adduct. It is important to remember that only the circled double bond can be chosen as a dienophile because of its proximity to the diene.
The next question we have to tackle is regio-selectivity. Since we are dealing with two different conjugated systems we start with the acrolein derivative. Drawing its canonical forms we observe that charges deposit on particular atoms in the system. This is illustrated below : (The phenyl ring is omitted for clarity and Ar denotes the ring system)
The same can be done for the other system as well : (The acrolein system is omitted for clarity)
Knowing that charges will accumulate at these sites we can draw a transition state that shows us which conformation the molecule will adapt for the formation of the Diels-Alder adduct:
From here the structure of the first precursor is apparent :
However, this precursor is not stable. The three membered ring that is fused with larger systems experiences a large amount of angle strain. A keen reader would now notice that two double bonds are conveniently placed along with one side of the three membered ring to form a cyclohexadiene ring. This cyclohexadiene system can participate in an electrocyclic ring opening to relieve angle strain. This opening generates the second precursor:
It should be apparent to the reader that the next step is a hydrogen shift. This occurs as during such a process both the furan ring and the benzene ring regain aromaticity. The highlighted hydrogen undergoes a [1,5] shift to generate the final compound :
Hence the three steps are:
Log in to reply
Awesome explanation!! :D
One doubt , how did you get the ring system charge distribution for 1st step?
Log in to reply
When the negative charge shifts into the cyclohexadiene system to give us two benzene rings.
Log in to reply
Ohk thanks
That's great! Would love to see more chemistry problems and develop the chemistry community!
Note that there are two fused benzene rings in the product contrary to what the figure shows. I hope to see a few mechanisms soon.
Log in to reply
Meaning?
Log in to reply
Along with the two benzene rings the other fused six membered diene should also be a benzene ring. I forgot to mention "fused" in the initial statement.
Log in to reply
Got it! :)
Next question in mechanism challenge ?
Log in to reply
Probably next week...
Log in to reply
Waiting eagerly! :D
try my chem problem ..only 1 solver till now :(
Log in to reply
And why don't you try the second challenge
Log in to reply
where is it ?
Log in to reply
here
Log in to reply
will try later today , seems tough :D
Log in to reply
I hope to see a few mechanisms!
So are you up for a challenge?
Log in to reply
may be , yes :D