Mechanism Challenge - 3

OK... This will probably be a monthly segment. I stumbled across this conversion and I thought that it was a really good way to test your understanding of cyclohexane. Do note that this question does not exceed the bar set by the JEE Advanced.
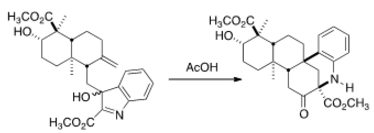
Account for the stereochemistry in your mechanism. A mechanism can be either hand drawn or made using an online web tool. An answer will be provided in 72 hours (or earlier) if no one can provide a suitable mechanism.
No vote yet
1 vote
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
Deducing the mechanism is quite a simple task. Comparing the product and reactant, we observe that the chemical formula is the same and hence acetic acid plays the role of a proton source. The nitrogen atom has a free lone pair, this is where protonation occurs.
The possibility of a six membered ring enables the double bond to attack the protonated nitrogen system:
The adjacent phenyl group participates to form a sigma complex :
Now the alcohol provides an electron push and regenerates the benzene ring and simultaneously forms a ketone. The product is formed.
With a basic overview of the mechanism, we observe that the stereochemistry of the lower ester group is determined in the second step. Since the protonated reactant and the reactant have the same molecular geometry, we must look into the conformation chosen by the reactant. To be thermodynamically stable, the reactant must have bulky groups in the equatorial position in chair form, and large conjugated systems must be planar. With these two requirements we can identify key regions (circled in red):
The carbon atom excluded in these regions determines the stereochemistry of the product. If the two groups are forced to line on the same plane, steric interactions would come into play. This forces a slight tilt above the plane to avoid the methyl steric interactions. When these observations are jotted down:
The reader should notice the 'hidden' twist boat conformer in the reactant. This accounts for the stereochemistry.
Log in to reply
Why didn't the -OH group get protonated first, it has lp of electrons too and even gives a good leaving group?