Naming Alkanes
What is ALKENES?
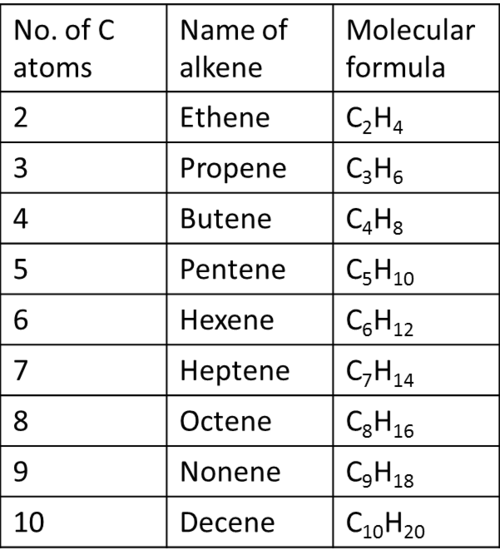
"Alkenes" contain* carbon-carbon double bonds* and are unsaturated hydrocarbons with the molecular formula is CnH2n. This is also the same molecular formula as cycloalkanes. Alkenes are named by dropping the -ane ending of the parent and adding -ene.
Steps of Naming ALKENES
STEP 1 * Name the main chain*
Find the longest continuous chain of carbons containing the double or triple bond The names of alkenes end with–ene, and the names of alkynes end with--yne. When there is more than one multiple bond, use numerical prefixes (diene, diyne, triene, etc.)
STEP 2: Number the carbon atoms in the main chain
Begin at the end nearer the multiple bond. If the multiple bond is at the same distance from both ends, begin numbering at the end nearer the first branch point.
STEP 3: Write the full name
Assign numbers to the branching substituents, and list the substituents alphabetically. Indicate the position of the multiple bond(s) in the chain by giving the number of the first multiple - bonded carbon. If more than one multiple bond is present, identify the position of each multiple bond and use the appropriate ending diene, triene, tetraene, and so forth.
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
There are no comments in this discussion.