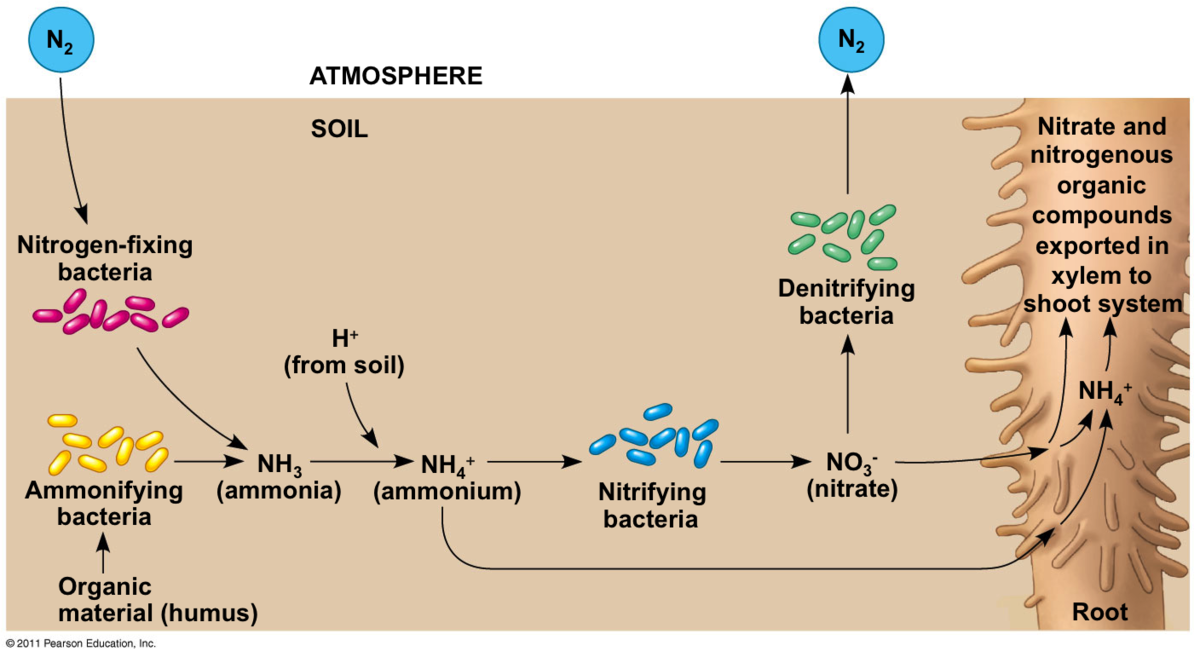
Nitrogen fixation
There is a certain class of fishes called bony fishes . As Ammonia passes through their gills , Ammonia gets converted to ammonium ions which then bony fishes will excrete as a waste product . As we know Ammonium ions are the substrate for the formation of nitrite (NO2^-) and nitrate (NO3^-), which is then taken up by plants thats when we call nitrogen has been fixed . But as we see Ammonium ions has been excreted by bony fishes , so Ammonium ions are there in water . Then we put the bacteria that oxidise ammonium ions into nitrites and then to nitrates . But as we know nitrates are soluble in water that means there will be no formation of precipitates . Nitrates will be there in water waiting for somebody to extract them and feed it them to plants so that by eating plants we can ultimately get that nitrogen . SO is there a way by which we can precipitate out nitrates which are originally soluble in water?
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
There are no comments in this discussion.