NSEP booster
Attention physics lovers!
Though NSEP is in November but i think its never too early. Therefore in this discussions we would try to cover as every concept at a stretch.
- Every week would have a topic for discussion and we would talk about every twist and turns in that topic so our journey to the mysterious island of physics start today.
I would also post questions in set named "That`s why I love physics" every week which I feel will definitely help.
i would like to include one thing out that the one who is good in one topic should help us out here.
No vote yet
1 vote
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
i invite @Archit Agrawal @Prakhar Bindal @Somyaneel Sinha ,@ Pankaj gupta @Gauri shankar Mishra ,@Shiv Agarwal ,@Jyotisman Das ,@Nihar Mahajan @Rajdeep Dhingra @Swapnil Das @avn bha ,@Abhineet Nayyar @Zerocool 141,@Anirban Mandal for participating in the discussion.
Log in to reply
Hey , All your mentions are wasted. Even I didn't receive mine. I wanted this thing badly. Let's get started.
I would like you to keep the topics related to circuits and electrodynamics.
Well @Aryan,I think i would like to join in to this stuff.I would really appreciate if you let me in.Since I haven't been active much,So if you could help me with things like where to start solving stuff? Thanks!
Log in to reply
definately you can join .anyone can take part in the discussion i just invited some people so that at least some people can get to know about it.
Log in to reply
new questions coming up?
Thanks for inviting me. Can we recommence?
--------FIRST LAW-----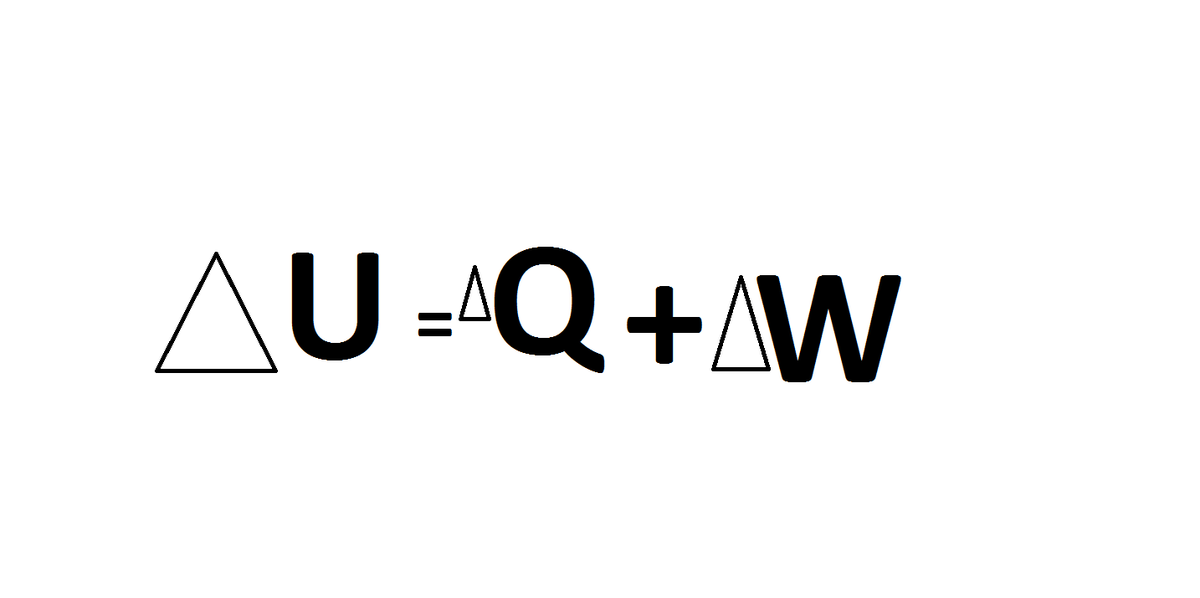 the base of thermodynamics lies in the above three alphabets,U,Q and W.
W-work done by the system(remember "BY")
Q-amount of heat given to gas
U-intenal energy
What is intenal energy if anyone could define?(there would be a session of cross-questioning and answering at 1:00 pm on monday which would help us to deepen our concepts and then i would post a question related to topic,so be ready with the answers )
the base of thermodynamics lies in the above three alphabets,U,Q and W.
W-work done by the system(remember "BY")
Q-amount of heat given to gas
U-intenal energy
What is intenal energy if anyone could define?(there would be a session of cross-questioning and answering at 1:00 pm on monday which would help us to deepen our concepts and then i would post a question related to topic,so be ready with the answers )
Log in to reply
Internal Energy Of Any System (Not necessarily an ideal gas ) can be simply defined as sum of the individual energy of each molecule (including kinetic,rotational,vibrational) in a frame wherein the centre of mass of the system is at rest
Log in to reply
Nice way of making center of mass at rest so as to express that we do not include energy due to external force field.
Now let us consider a case in which there is a container containing an ideal gas ,two situation are given state what is the change in internal energy using defination of it. take all the walls as adiabatic including the piston one.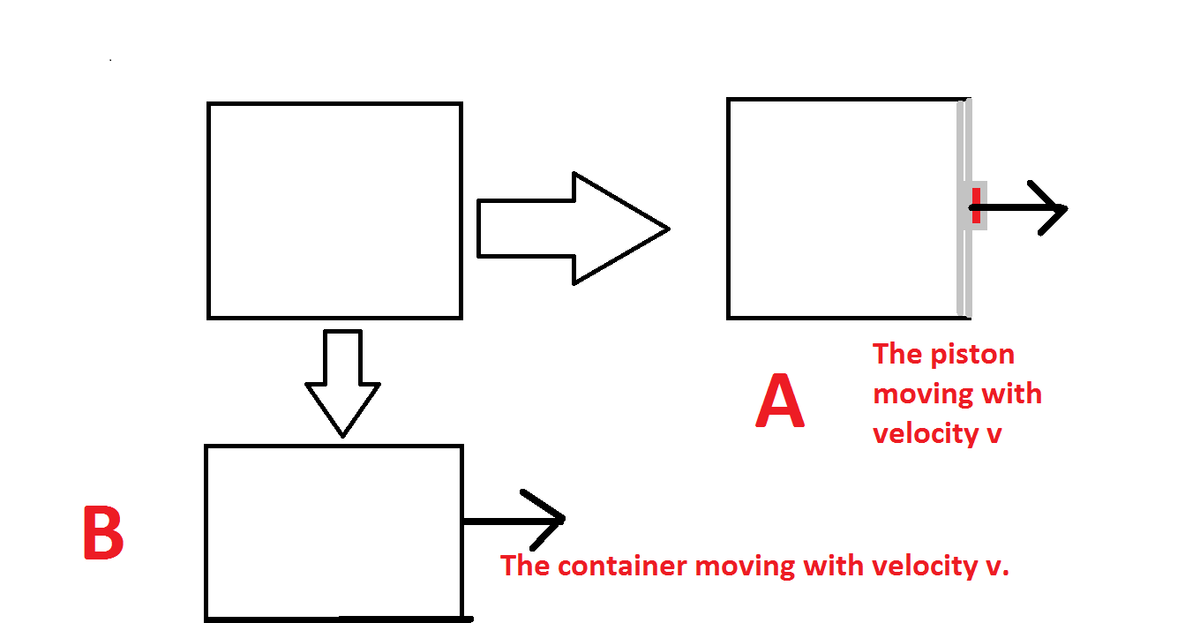
Log in to reply
Well If a container is moving i think velocity wrt centre of mass will not change and hence there (molecules) energy will also not change. i think Change in internal energy = 0
Log in to reply
very true, since you had already added in the defination that we see energy in center of mass frame the both initial container and container in case B is in same situation.
Log in to reply
Now i will analyse case A (i think it will be a bit harder than case b) but still i will give it a shot
Also i would like to add that temperature of gas will remain constant in case B Because internal energy of an ideal gas is only and only a function of temperature. Also if the gas was a real one that If internal energy remains constant then its not necessary that temperature will remain constant because for real gases U Depends on many other factors too
Log in to reply
thanks for this insight , but may you please list all the other factors in real gas case.
Log in to reply
Any thing that will change the intermolecular forces And intermolecular separations will change the internal enrgy of a real gas because Potential energy of molecules will change .Also as far as i know According to KTG For an ideal gas the intermolecular forces of attraction are neglected in case of ideal gases And hence i think These factors do not affect the internal energy of an ideal gas.
Any factor that will change a and b (vanderwaal's constants) will also alter internal energy
a is a measure of intermolecular force of attraction and b is responsible for excluded volume of a real gas
i also found a quite good piece of information at this link
http://public.wsu.edu/~scudiero/documents/Homework3-hints.pdf
Log in to reply
thanks for the explanation and the link.
Log in to reply
Hey will internal energy decrease in case A. If its correct then will add an explanation
Log in to reply
case A or B
Log in to reply
Its A Sorry
internal energy will decrease.
Log in to reply
Ok Here is my explanation.Firstly as the process is adiabatic the net heat exchange with surroundings would Be Zero .Next using first law of thermodynamics Q = U + W ( There are change in internal energy and work done by the system).
We must have U+W = 0 . As the piston is moving then the volume of the gas will increase (gases have sufficient entropy to enclose whole of the volume they are put in). Hence work done by the gas would be postive (W = P(V2 - V1) ) .Now As W Is positive U Will be negative.
Log in to reply
nice one but let me tell you tell you one thing that the solution to this can be drawn without using first law and using your own defination.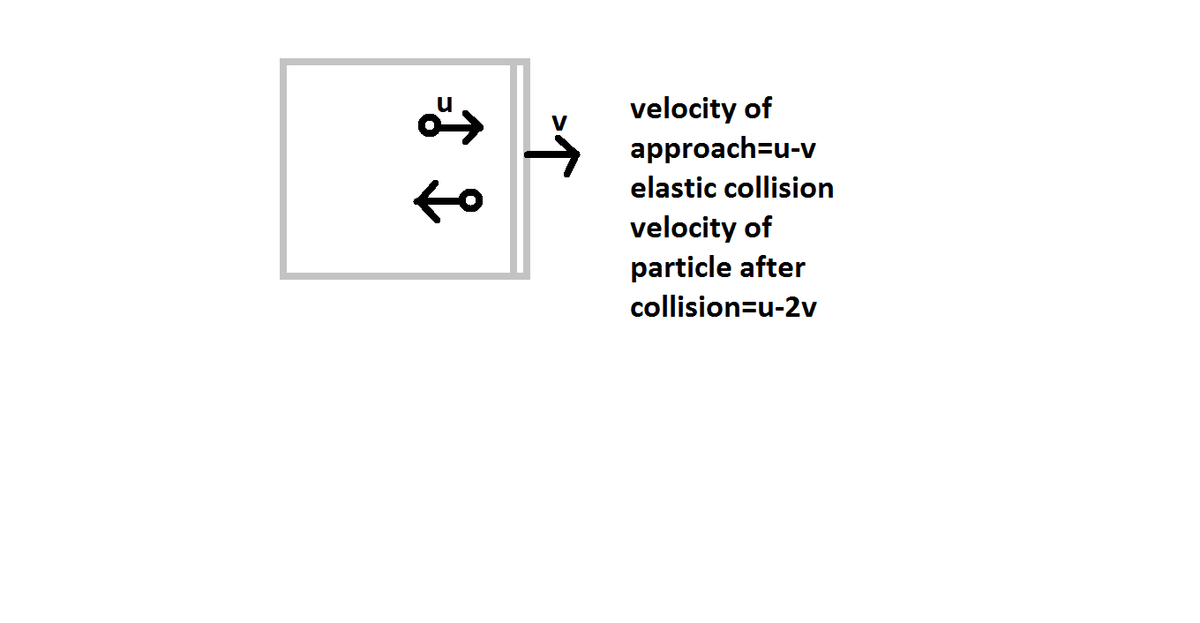 so internal energy decreases
so internal energy decreases
Log in to reply
Oh brilliant! +1!
Log in to reply
the temperature in case B decreases as internal energy decreases and it is an ideal gas. Now can you tell why temperature and internal energy are related.
Log in to reply
i don`t know how all the energy that constitute the internal energy depend on temperature but yes translational kinetic energy depends on temp {by KTG}=3/2{nRT} This would get you proff http://teacher.pas.rochester.edu/phy121/lecturenotes/Chapter18/Chapter18.html added to this
What is difference between internal energy of ice and water(both at same temperature ie O degree celcius)?
I Will Analyze And Report !
Log in to reply
so now when you have to look for internal energy just sit in cm and then analyze ,answer would lie with you remember internal energy in dependent only on temperature in case of ideal only not in real , i would come to that point when we will come in mathematical analysis of thermo.
--------FIRST LAW-----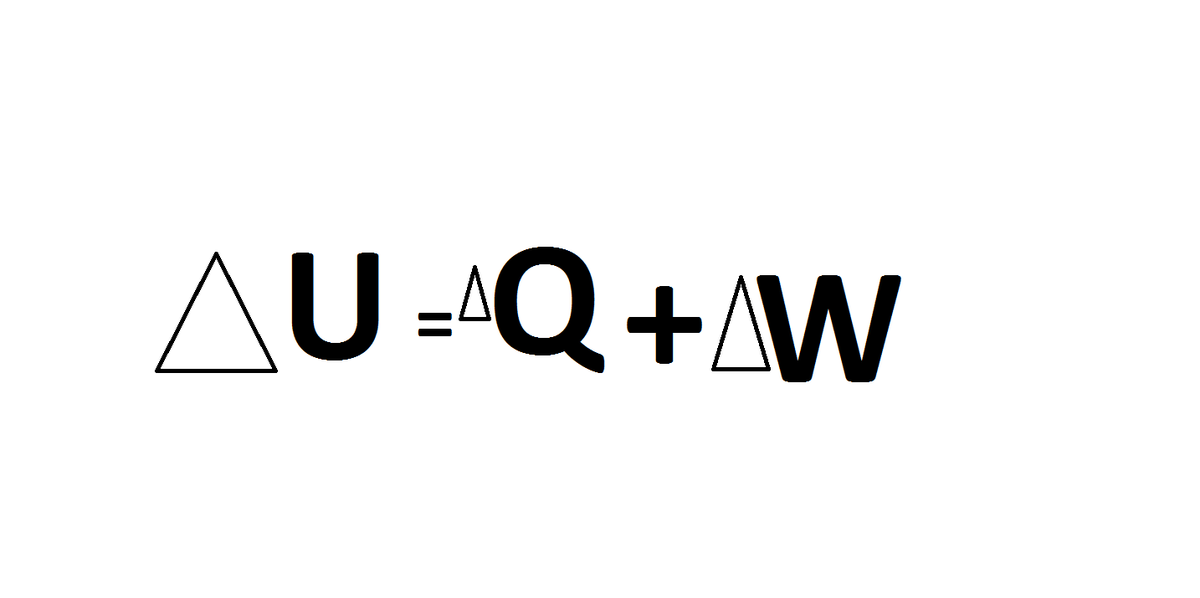 the base of thermodynamics lies in the above three alphabets,U,Q and W.
W-work done by the system(remember "BY")
Q-amount of heat given to gas
U-intenal energy
we already discussed about internal energy in our previous session and it`s more than clear now.
What is "W-work done" if anyone could define and on what factors does it depend?
the base of thermodynamics lies in the above three alphabets,U,Q and W.
W-work done by the system(remember "BY")
Q-amount of heat given to gas
U-intenal energy
we already discussed about internal energy in our previous session and it`s more than clear now.
What is "W-work done" if anyone could define and on what factors does it depend?
Log in to reply
But i want more people to participate not only just 2 people
to deepen the concepts of work let us consider the situation.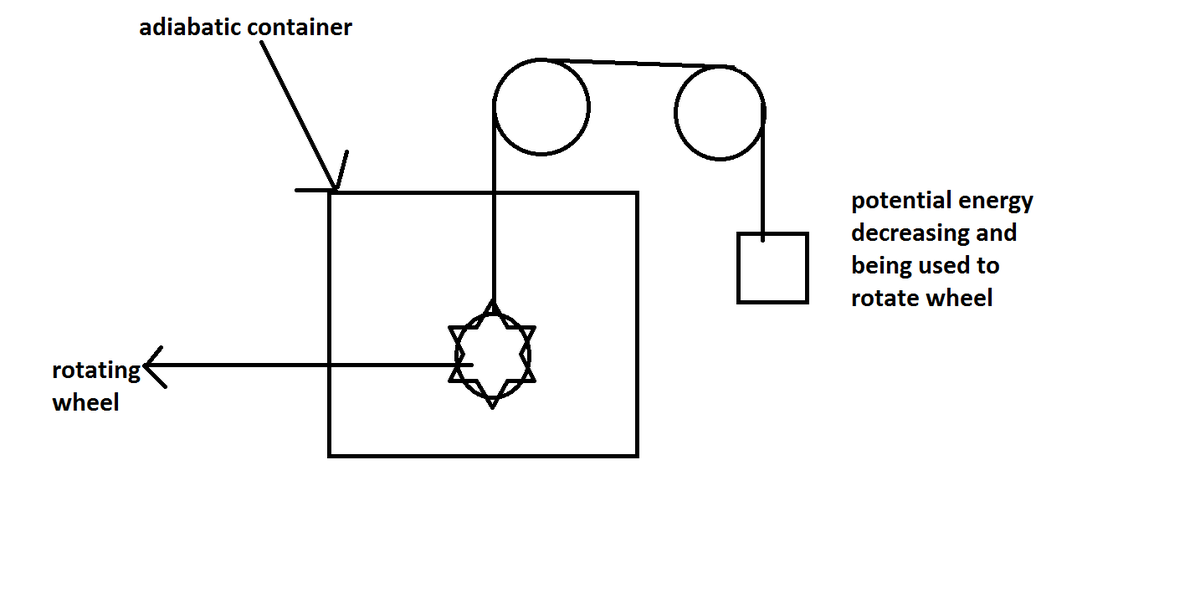 state in this case what will be zero
heat supplied or work done by the system and why?
state in this case what will be zero
heat supplied or work done by the system and why?
Aryan invites @Archit Agrawal @Somyaneel Sinha @Jyotisman Das @Abhineet Nayyar @Zerocool 141 @Anirban Mandal
Log in to reply
hey thanks ! you should first read the underwritten text and then try my 2 questions on internal energy in set "that`s not why i love physics" and then we will begin with discussion on work.
Log in to reply
Most people have exams currently. 21st March is mostly end date for people's exams. We'll start then.
Log in to reply
ok it`s fine then i would change the date declared.
Thank You for inviting me. I will definitely take part in it once my board exams are over. It will end on 15th of March.
Log in to reply
You're in 10th? Boards?
Log in to reply
Sorry. I meant Eleventh Final Exams.
When are we starting. We should start @aryan goyat @Gauri shankar Mishra @Anirban Mandal
Log in to reply
Exactly. I am seriously impatient.
now only.
Log in to reply
Hey should we start with mechanics?
please choose a topic for discussion.
Log in to reply
Mechanics!
I Invite @Aniket Sanghi Too
Log in to reply
Discussion is here or somewhere else
Log in to reply
On Slack.
Log in to reply
how to open it?? i am unable to open
Log in to reply
go to click here. Login by entering your email and all.
Are all of you in Slack Chat ? If not join by clicking here. After you all have joined leave a reply to this comment.
Log in to reply
@aryan goyat@Gauri shankar Mishra @Jyotisman Das @Ayush Agarwal @Aniket Sanghi @Somyaneel Sinha @Archit Agrawal @Anirban Mandal @Shiv Agrawal
Log in to reply
I have already added @Gauri shankar Mishra@Aniket Sanghi@Prakhar Bindal@Swapnil Das@Adarsh Kumar to the channel on slack. Rest of you inform me when you join slack.
Log in to reply
I am in too!!
i have joined
Log in to reply
I have added you.
Which channel
Log in to reply
nsepbooster , it is a private channel. I have added you in it.
Log in to reply
i have posted the very first ques for practice of mechanics NSEP titled elliptical cage though its little mathematical.
Log in to reply
I saw. I was working on it. Thanks
I have joined it.
Log in to reply
aaj aa raha hai.
I have added you in the channel.
part-2 so here is my next ques 'may-be-this-is-more-easy-then-you-think' you can find it my set that's why i love physics
here is my 3rd problem 'can you do it in 3 minutes'
Can someone please tell me the cuttoff of nsep which was held on 22 november !!!!!
Log in to reply
different for different states.
Log in to reply
U know for UP
@puneet manglaPlease give some advice for NSEP. Also what is the name of your book ?
Log in to reply
i know him its just a title and the ques are from different CPP,books and class problem .
Ok guys lets start with Level 2 one i mean INPHO.Check out my newest post .
Log in to reply
You qualified NSEP? What were your marks?
Log in to reply
170 something just qualified :P.
Thanks for inviting me! I would love to join the discussion. I am good at application part of first law, so I may be of help.
i have posted 1st ques for nsep in set 'that,s why i love physics' all of you should try it it title is 'Elliptical cage'