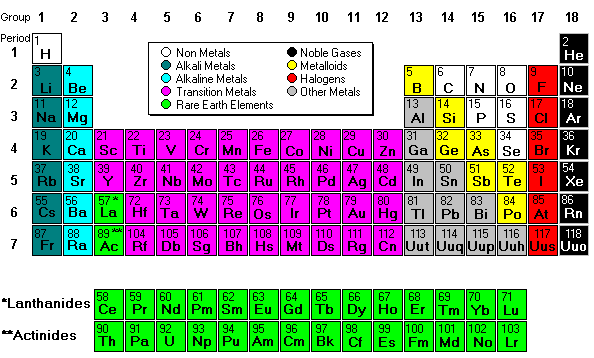
Periodic Table I - Chemistry
The Periodic Table Is A Tabular Arrangement of the Chemical Elements, Organized on the Basis of Their Atomic Numbers, Electron Configurations (Electron Shell Model), and Recurring Chemical Properties. Elements Are Presented in Order of Increasing Atomic Number (the Number of Protons in the Nucleus). The Standard form of the Table Consists of A Grid of Elements Laid Out in 18 Columns and 7 Rows, With A Double Row of Elements Below That. The Table Can Also Be Deconstructed Into Four Rectangular Blocks: the s-block to the Left, the p-block to the Right, the d-block in the Middle, and the f-block Below That. The Rows of the Table Are Called Periods; the Columns are Called Groups, With Some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences.
Groups or Family
Is a Column of Elements in the Periodic Table of the Chemical lements. There are 18 numbered groups in the periodic table, but the f-block columns (between groups 2 and 3) are not numbered. The elements in a group have similar physical or chemical characteristic of the outermost electron shells of their atoms ( the same core charge), as most chemical properties are dominated by the orbital location of the outermost electron.
 Table
Table
Periods
In the periodic table of the elements, elements are arranged in a series of rows (or periods) so that those with similar properties appear in a column. Elements of the same period have the same number of electron shells; with each group across a period, the elements have one more proton and electron and become less metallic. This arrangement reflects the periodic recurrence of similar properties as the atomic number increases. For example, the alkaline metals lie in one group (group 1) and share similar properties, such as high reactivity and the tendency to lose one electron to arrive at a noble-gas electronic configuration. The periodic table of elements has a total of 118 elements.
The first period contains fewer elements than any other, with only two, hydrogen and helium. They therefore do not follow the octet rule. Chemically, helium behaves as a noble gas, and thus is taken to be part of the group 18 elements. However, in terms of its nuclear structure it belongs to the s block, and is therefore sometimes classified as a group 2 element, or simultaneously both 2 and 18. Hydrogen readily loses and gains an electron, and so behaves chemically as both a group 1 and a group 17 element.
Hydrogen is the most abundant of the chemical elements, constituting roughly 75% of the universe's elemental mass.[1] Ionized hydrogen is just a proton. Stars in the main sequence are mainly composed of hydrogen in its plasma state. Elemental hydrogen is relatively rare on Earth, and is industrially produced from hydrocarbons such as methane. Hydrogen can form compounds nt in water and most organic compounds.
Helium exists only as a gas except in extreme conditions. It is the second lightest element and is the second most abundant in the universe. Most helium was formed during the Big Bang, but new helium is created through nuclear fusion of hydrogen in stars. On Earth, helium is relatively rare, only occurring as a byproduct of the natural decay of some radioactive elements. Such radiogenic helium is trapped within natural gas in concentrations of up to seven percent by volume.
Period 2 elements involve the 2s and 2p orbitals. They include the biologically most essential elements besides hydrogen: carbon, nitrogen, and oxygen. Lithium (Li) is the lightest metal and the least dense solid element. In its non-ionized state it is one of the most reactive elements, and so is only ever found naturally in compounds. It is the heaviest primordial element forged in large quantities during the Big Bang.
Beryllium has one of the highest melting points of all the light metals. Small amounts of beryllium were synthesised during the Big Bang, although most of it decayed or reacted further within stars to create larger nucleii, like carbon, nitrogen or oxygen. Beryllium is classified by the International Agency for Research on Cancer as a group 1 carcinogen. Between 1% and 15% of people are sensitive to beryllium and may develop an inflammatory reaction in their respiratory system and skin, called chronic beryllium disease.
Boron does not occur naturally as a free element, but in compounds such as borates. It is an essential plant micronutrient, required for cell wall strength and development, cell division, seed and fruit development, sugar transport and hormone development , though high levels are toxic.
Carbon is the fourth most abundant element in the universe by mass after hydrogen, helium and oxygen and is the second most abundant element in the human body by mass after oxygen, the third most abundant by number of atoms. There are an almost infinite number of compounds that contain carbon due to carbon's ability to form long stable chains of C—C bonds. All organic compounds, those essential for life, contain at least one atom of carbon; combined with hydrogen, oxygen, nitrogen, sulfur, and phosphorus, carbon is the basis of every important biological compound.
Nitrogen is found mainly as mostly inert diatomic gas, N2, which makes up 78% of the Earth's atmosphere. It is an essential component of proteins and therefore of life.
Oxygen comprising 21% of the atmosphere and is required for respiration by all (or nearly all) animals, as well as being the principal component of water. Oxygen is the third most abundant element in the universe, and oxygen compounds dominate the Earth's crust.
Fluorine is the most reactive element in its non-ionized state, and so is never found that way in nature.
Neon is a noble gas used in neon lighting.
All period three elements occur in nature and have at least one stable isotope. All but the noble gas argon are essential to basic geology and biology.
Sodium is an alkali metal. It is present in Earth's oceans in large quantities in the form of sodium chloride (table salt).
Magnesium is an alkaline earth metal. Magnesium ions are found in chlorophyll.
Aluminium is a poor metal. It is the most abundant metal in the Earth's crust.
Silicon is a metalloid. It is a semiconductor, making it the principal component in many integrated circuits. Silicon dioxide is the principal constituent of sand.
Phosphorus is a nonmetal essential to DNA. It is highly reactive, and as such is never found in nature as a free element.
Sulfur is a nonmetal. It is found in two amino acids: cysteine and methionine.
Chlorine is a halogen. It is used as a disinfectant, especially in swimming pools.
Argon is a noble gas, making it almost entirely nonreactive. Incandescent lamps are often filled with noble gases such as argon in order to preserve the filaments at high temperatures.
What should I improve ?
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
Ar(18) lies to the left of K(19) although it's atomic weight is 39.948 to 39.098. Same goes with Co(27) and Ni(28) with their atomic weights of 58.993 and 58.693 , and Te(52) and I(53) with their atomic weights of 127.603 and 126.904 respectively. Can you find other pairings where one of its pair has a higher atomic number but a lower atomic weight? (Hint: Check the actinides)
Log in to reply
Hey Venture, i don't know if i am correct but..we all know about Mendeleev's periodic table...it was rejected as he had placed elements of greater atomic mass before the ones with lesser atomic mass...i was reading his table the other day and compared it to the modern periodic table and i found out that actually he had placed them correct, it was his calculation error to have miscalculated the atomic mass of some elements...i need someone to confirm if i am right...please can you help?
Log in to reply
It might have something to do with how they calculate the atomic weight of each element using a weighted average weight among the isotopes of the same element. For instance, Co(27) has one proton less than Ni( 28) but the average atomic weight for Ni ( and its isotopes) is slightly less than the average weight for Co and its isotopes.
Log in to reply
okay..thnx venture for the clarification
Nice ! :D
Nice my atomic number is 10 and I am neon