Physical Chemistry - Electrochemistry - 3
Before reading this post, please read the first two posts here :
Physical Chemistry - Electrochemistry - 1
Physical Chemistry - Electrochemistry - 2
ELECTROCHEMICAL SERIES
 alt text
alt text
It is series where elements are arranged in increasing order of Standard Reduction potential (or decreasing order of Standard Oxidation potential) as compared to that of S.H.E. It is experimentally determined.
Some General Characteristics of this series are:
Elements near the top of the series are highly electropositive. They lose electrons readily to form cations.
Elements near the bottom of the series are highly electronegative. They gain electrons readily to form anions.
Note that, the Standard Reduction Potential of Hydrogen is zero.
Keeping these points in mind, we can draw some general conclusions, such as,
If metal A is situated above metal B in the electrochemical series, then A can displace B from its salt. The reason for this is that, since , implying that , thus suggesting that A has more tendency to form cations than B.
Metals situated above Hydrogen, are easily rusted. This is because these metals will have a higher tendency to oxidize (tendency to form cations). And since the definition of rusting is oxidation of metal, these metals will rust easily.
Before we can learn how to use this series to calculate the EMF of cells, we need to understand how to represent a cell as an equation. We cannot write long sentences to represent a cell everytime. It is time-consuming, and also unnecessary.
NOTATION FOR REPRESENTING A GALVANIC CELL
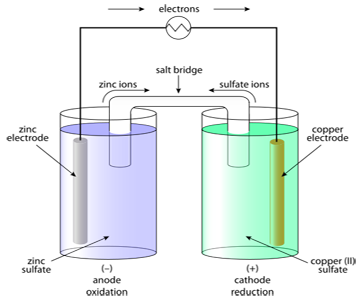
First of all, a Galvanic cell works through a redox reaction. One metal is oxidized, while another metal's cation is reduced.
I think it will be best to approach this idea through an example.
For eg, consider the cell which uses the following reaction :
The interfaces, across which a P.D exists, are shown by a vertical line ( | ). In this case, the two half cells are represented as,
and
These indicate that a P.D. exists at the and ions interface, and similarly at the and interface.
Sometimes, a comma is observed in the formation of the half cells. For example,
These indicate that and together constitute the electrode.
The contact between two solutions by means of a Salt Bridge (will be explained later) is indicated by double vertical line (||) between them. For example,
The anode half-cell (oxidation half-cell) is always written on the left hand side, and the cathode half-cell (reduction half-cell) on the right, with the respective metal electrodes on the outside extremes. For example,
The concentration of solution, pressure of gases and physical state of solids and liquids involved are indicated in the cell formation. For example,
In the next post, we shall learn how to calculate the standard EMF of any cell, with the help of our new tool : Electrochemical Series.
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
Completely agree with Anish. Can't thank Brilliant enough!!
It will take a while to post the next note, since I have my 12th grade's Final Board examinations coming up, which will end at 14th March, 2014.
I will post my next note, a few days after this date. Sorry guys.
Log in to reply
Good luck on your Boards Anish! Let us know if there is anything people on Brilliant could do to help you prepare.
Log in to reply
I have already learned too much here at Brilliant. This site is like a god gift to a student who is preparing for an entrance exam. Thank you so much (I cant tell how much) for your help..Its been a wonderful journey so far.!
Log in to reply
Anish, Best of Luck for your boards. I also have my 10th grades finals coming up on March 3 and they end on March 26.
Log in to reply
Good luck to you as well, do it nicely.
btw its Anish, not Danish..lol
Log in to reply
Sorry Anish that was not intentional and thanks a lot.
Log in to reply
Its ok Soham.
Log in to reply
Ha ha ha ha, that's funny.
Well written. Great post!
a redox reaction is carried out at 400k. If the same reaction is carried out in presence of a catalyst at the same temperature the rate of reaction doubles . To what extent is the energy barrier loweredby the catalyst . [Use R=2cal/mol-k and log2=0.301] (a)693cal (b)554cal (c)455cal (d)231cal
Nice work there