Please help.
amount of heat is given to mole of an ideal mono atomic gas by processes
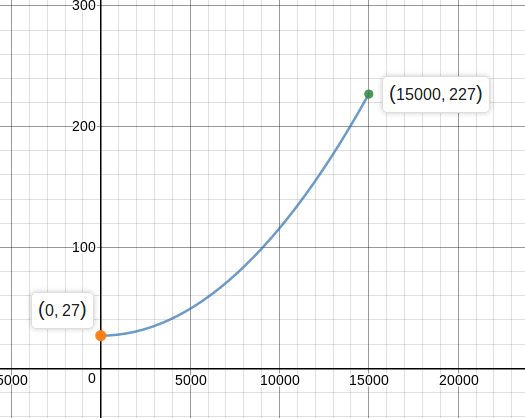
Following graph shows variation of temperature with . Find the value of .
No vote yet
1 vote
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
We know that dQ=nCdT. Since the amount of heat exchanged and temperature are both state functions and depend only on the process, we can also say that ΔQ=nCΔT. Thus
C=n1ΔTΔQ=0.51227−2715000−0=150J/K
If the gas undergoes the process PVx=k, then heat capacity for the process is given by
C=R(γ−11+1−x1)
For TVn=k, we have PVn+1=k and thus
150=R(35−11+−n1)⟹n1=23−R150
What is the unit of heat supplied?
Log in to reply
Yes, you are right R=325 which yields correct result. Thanks :)
Log in to reply
@Tapas Mazumdar But a doubt still persists, that when the process is coming out to be in the form of PVx=k isn't it adiabatic?
Log in to reply
As you can see from the above table only when x=γ, the process is adiabatic. In general any process of this from is a polytropic process*.
Log in to reply
Okay, have to be careful next time.
Log in to reply
I'm glad your concepts got clarified. :D