Stretching Vibrational Frequency in Coordinate Compounds
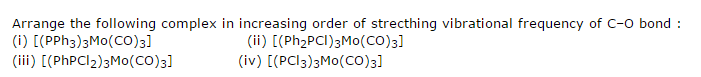
My Intellectual Friends,
I came across this today, actually now in fact.
What is this? How do I approach this question?
No vote yet
1 vote
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
@@Tanishq Varshney
Log in to reply
Share with others too dude.
from where did you found this ? As this is not in JEE Syllabus
Log in to reply
I found this in one of my mock tests. By Allen
Perhaps you may google out for Carbonyl Complexes and their properties
Log in to reply
I searched so. I even searched for Stretching Vibrational Frequency and also found a Wikipedia page. Unfortunately, couldn't understand much. So I posted it here.
Carbonyl's antibonding molecular orbital(pi) can form a back bond with a filled t2g metal orbital. All this means is that when the back bond is weakening, the frequency will increase.
Hence one must compare the compounds and see if the different ligands can affect the back bond. The P-Phenyl ABMO is of comparable energy with the filled t2g metal orbital. Chlorine takes away most of Phosphorus's electron density, hence the metal isn't very rich in ions. This isn't the case with phenyl, they allow phosphorus to give electron density to the metal and hence increase chances of backbonding. Ligands that increase the chances of back bonding between the metal and the carbonyl decrease frequency. This happens as back bonding lowers the bond order which lowers frequency.
So I think the order will be (iv) > (iii) > (ii) > (i)
This is an interesting question. Do you know the answer?