The moving or not moving neutrons.
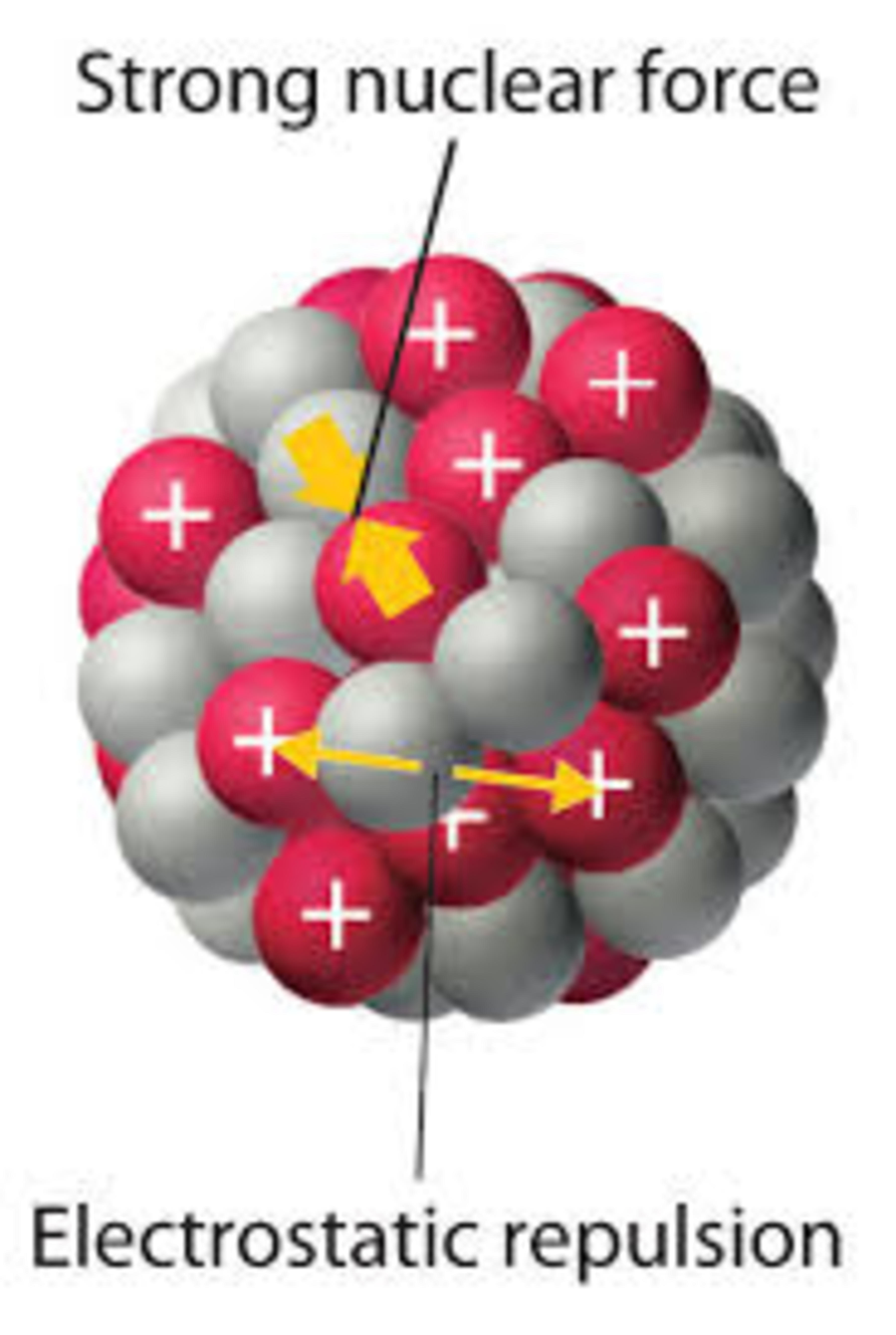
Consider an atom where you have a bunch of neutrons and protons in the nucleus. The electrons appearing/disappearing from here to there, spinning or doing whatever around the nucleus. Now we know neutrons don't get affected by the EM force and the nuclear force is much greater than the EM force, so the question arises how does our hand or any other object move? ( Not considering the motion of objects due to gravity, Also the hydrogen atom which doesn't consist the neutrons ). For example, A person pushing a table which fundamentally is the EM force.
 Source: Google
Source: Google
So if we look the EM force (electrons repelling) from our hand affects the electrons of the table which cause them to move and then similarly the protons following the electrons. But now the neutrons don't get affected by the EM force and also the nuclear force is much greater than the EM force and thus shouldn't the protons actually rather than following the electrons, should stay glued to the neutrons which results in the nucleus not moving and thus following the whole atom not moving. So the question is How does an atom move under the influence of the EM force?
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
I think I have a rather simple solution to this question : the neutrons aren't affected by EM force, but nuclear force isn't holding them in place. Therefore when protons move, neutrons follow, because they are glued to them. The only thing maintaining the neutrons in place (and in fact everything, in this case) is inertia, which isn't that great for tiny particles.
Log in to reply
Would you mind if you could show the calculation with some pictures or showing the motion for a nucleus of a real atom? For example, Lithium
There are several things going on here that we need to pay attention to. First of all, while it is true that the interactions going on while you push the table are essentially a manifestation of the EM force, it's not a straight repulsion of similar charges. When you place your hand on the table to push our pull it, there is no net transfer of electrons that would cause large scale repulsion. If there was, how would we explain our ability to pull the object? It's important to remember that all the atoms are bound together through both chemical bonds and also the bonds holding molecules together. All of these internal forces are also at play.
Second, we need to take a look at Newton's second law. Before we push the table, all external forces on the individual atoms are balanced, since the atom is currently in a state of rest (let's ignore the vibrating for now). In addition, all internal forces inside the atom must also be balanced, or else the atom would rip apart. Each proton is getting repelled by every other proton, but it is also being held by the strong force from the protons and neutrons. So the sum of forces is zero. That means some of the neutrons are pushing the protons left, right, up, down, and every other direction. A net external force resulting from our push does not change this state of internal balance and so, even though the neutrons don't experience the EM force, the protons would still drag them along. The neutrons aren't anchored in place and the protons pull on the neutrons with just as much force as the neutrons pull on the protons (Newton's third law). There's no way the neutrons could stop the resulting movement.
It is somewhat similar to pushing a stack of books. We don't need to apply the force to every book in the stack because we rely on the friction to hold the stack together, just as we expect the strong force to home the atom together.
Log in to reply
I am still a little not convinced. Would you mind if you could show me a more elaborate calculation of a nucleus, for example, the nucleus of lithium as it has odd number proton and neutrons? ( 3 protons and 3 neutrons)
Log in to reply
Maybe an analogy could help a bit.
Imagine a billiard ball on a pool table. It's made from acrylic and it's pretty hard to rip apart, you'd certainly need a few tools if you wanted to do so. You can see this as the nucleus being hold together by the strong force.
Yet, you don't need strong tools to change the motion of the ball. A small tap from your finger will make it have some velocity. You can see this as the external electromagnetic forces applied upon the nucleus. If you hit the ball harder, it will fly over the other side of the table with greater velocity. But whatever the force you apply, I'm sure you can see it will never be enough to pry open the ball. It will conserve its integrity, unless being applied upon it forces several order of magnitude greater.
I think your confusion comes from the fact that you're likely picturing the billiard ball, made from acrylic, as the same material as what the green is made of, and that instead of thinking about a point of contact between the ball and the table, you're thinking about a continuous link of matter between the two. Indeed if the ball was but a simple extension of an acrylic table, you couldn't move it by just pushing it lightly. The link of matter between the table and the ball would prevent you to do that, and you'd need forces able to break that link in order to apply any velocity on the ball.
Inside the nucleus, neutrons applies a great force upon protons, but don't apply that force upon the fabric of space time (which is the green of the pool table in the analogy). Hence, smaller forces can make them move (a light tap of your finger).
Hope that helps.
Log in to reply
In the real level, even analogies seem to fail. Now imagine if there is an EM force on a proton towards the left side and if a neutron is in front of it, would the pair actually move as the proton cannot apply a force of push on the neutron like if one solid sphere pushes another sphere in front of it because there is no force like EM force which pushes another solid sphere, in the proton-neutron scenario.
Log in to reply
"[A] proton cannot apply a force of push on the neutron"
But it does. Otherwise how would even a deuterium nucleus be stable? If neutron and protons don't push each other, they are bound to collapse into one another.
Log in to reply
Then if they are pushing each other what force is it? I mean if I don't know any theory please explain.
Log in to reply
If I understand correctly, it's the strong force. The strong force does bind quarks together and does bind protons and neutrons together, such as to form a nucleus. But it's also repulsive so they don't collapse onto each other. I know it sounds weird, the strong force isn't as straight forward as gravity.
For instance, it's the reason why neutron stars don't collapse into black holes. Since they are perfectly neutral clumps of matter, no EM force could be responsible for pushing protons (and thus nucleus) away from each other. It's just neutrons, crammed next to one another. And the strong force keep them from undergoing fusion.
Log in to reply
Ok. But I see some other interactions and some other stuff for hadrons floating around the internet. I understand what you mean to say. But if you write a nice comment with a proper model which is accepted widely around the globe with the help of some pictures or links I would appreciate it.
Log in to reply
I understand your need for a clear explanation, but I'm not the one able to provide it. I'm only starting my journey on the subject :)
Have you read the relative wiki pages? It could be a good starting point. And then with the references and bibliography you're unstoppable.
Log in to reply
Yes even I have started studying about quantum physics or rather nuclear force a year ago, so this was the only major doubt I had. I'm still understanding nuclear physics. I will see if somebody can write a clear solution also.
Log in to reply
Hey Siddharth, there is mention of what we're discussing in the explanation of this problem: https://brilliant.org/practice/coulombs-law-2/?problem=electricity-and-magnetism-problem-161518&chapter=introduction-14
I thought it might be of interest to you. Cheers
@Siddharth Chakravarty How a nucleus is stable??How protons are present in a small space of nucleus??
The neutron dissociates into a proton and a meson (a negatively charged particle).Therefore protons get attracted toward neutron,but wait there is a proton also.So,strong force is attractive as well as repulsive.So,proton don't collapse into the neutron.But,a question arises if a neutron is behind a proton,then it can't move forward due to strong force,but wait we ain't sure about position because micro particles are wave also.So,at a same time it is present in all nucleus with different probability magnitudes.Hence force is acting on a proton in all direction and nucleus is stable.Hence resultant of all forces must be zero and when we apply em force on atom then it moves forward (because neutron are also combination of proton and meson)
Hope it helps!!!
Feel free to figure out any mistake.
Log in to reply
Your answer seems a bit plausible, but to verify it, could you please provide any relevant data or link on the topic. I know about mesons and particles are said to have wave functions about their positions but not referred to as waves as you mentioned, but I am asking for data which answers this question in a way. Although, this is an open discussion.
Log in to reply
After 0.7 fm nuclear force becomes repulsive. And i have just give a intution and i will try to take other complex mathematical computations...(but,i don't langrage multipliers:-P😅) and every particle is referred as a wave so,that they have wave functions. The electron and nucleons are nothing but the probability functions.
@Siddharth Chakravarty,have you found sonething.
Log in to reply
Still researching new things!
nuclear force, yukawa's potential,wave particle duality
these are few links,i have not understand them whole, but something comes to my mind and i gave answer... @Siddharth Chakravarty
Log in to reply
I want somebody to give the answer who can correctly answer this as we all can interpret realities on what knowledge we have now. But I hope an answer from somebody who has the latest and correct knowledge till now what mankind knows about the quantum world. You may be right :)
Log in to reply
Then not only we have to focus on matter and energy but also on free space Quantum fluctuations.
Quantum fluctuations.
Log in to reply
But I think we will wait till somebody says with full confidence I guess, but your answer is also something I will consider. Thanks!! We don't know a lot of things about the quantum world for now, or might never...
Log in to reply
Yes.......