Type of Heat
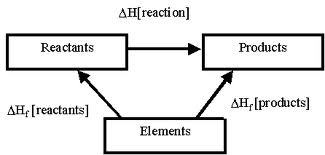
1)HESS'S LAW
Definition of Hess's law : states that the heat evolved or absorbed in a chemical process is the same whether the process takes place in one or in several steps. This is also known as the law of constant heat summation.
2)Heat of Combustion
Definition :Heat of formation is defined as the enthalpy change when one mole of a compound is formed from the elements in their stable states. For example, the heat of formation of water vapor is defined by the reaction:
Formula : Heat of reaction = [the sum of all heats of formation of all products] - [the sum of all heats of formation of all reactants]
3)Heat of Neutralization
Definition: Large concentrations of hydrogen ion and hydroxide ion cannot coexist in solution, because the neutralization reaction
H+ (aq) + OH- (aq) → H2O (l)
strongly favors the product (water).
4) Heat of Reaction
Definition ~ the heat evolved or absorbed during a chemical reaction taking place under conditions of constant temperature and of either constant volume or more often constant pressure
~ the quantity involved when gram equivalents of the substances enter into the reaction
5)Heat of Formation
Definition :the heat evolved or absorbed when one mole of a compound is formed from its constituent atoms
Easy Math Editor
This discussion board is a place to discuss our Daily Challenges and the math and science related to those challenges. Explanations are more than just a solution — they should explain the steps and thinking strategies that you used to obtain the solution. Comments should further the discussion of math and science.
When posting on Brilliant:
*italics*or_italics_**bold**or__bold__paragraph 1
paragraph 2
[example link](https://brilliant.org)> This is a quote# I indented these lines # 4 spaces, and now they show # up as a code block. print "hello world"\(...\)or\[...\]to ensure proper formatting.2 \times 32^{34}a_{i-1}\frac{2}{3}\sqrt{2}\sum_{i=1}^3\sin \theta\boxed{123}Comments
There are no comments in this discussion.