A to Z Chemistry (Finding unknowns)
Warning: This question is pretty long. Treat it as a puzzle rather than a problem. At the end problem is simple but you have to solve for all the unknowns to crack the answer. Please don't try to guess the answer. You will enjoy finding the unknowns. I have provided hints at the end.
Part-1
A metal ( A ) is reacted with ( B ) (a neutral oxide, liquid at room temperature), gives off ( C ) and ( D ), and ( D ) burns to give ( B ). ( C ) on heating at around gives ( E ) and ( B ). ( E ) when treated with ( F ) in electric arc furnace gives ( G ) and ( H ). ( H ) is an extremely poisonous, tasteless, odorless, colorless gas that burns with a blue flame. ( G ) on treatment with ( B ) gives ( I ) and ( C ). ( I ) is a highly flammable gas and decolorizes bromine water. ( I ) on heating in red hot copper tube trimerises to form ( J ) as the major product.
Part-2
Another metal ( K ) reacts with ( F ) in an electric arc furnace at about to to form ( L ). ( L ) reacts with ( B ) to form ( M ) and ( N ). ( N ) is basic in nature and sparingly soluble in water. ( M ) reacts with one equivalent of greenish yellow gas ( O ) in the presence of UV light to give ( P ). ( O ) also reacts with ( D ) in the presence of UV light to give ( Q ), aqueous solution of which reacts with ( N ) to give ( S ) which is a lewis acid and fumes in moist air.
Part-3
( J ) is reacted with ( P ) with ( S ) as a catalyst to give ( T ) which when treated with conc. gives ( U ). ( U ) is again treated with ( P ) with ( S ) as a catalyst to give ( V ). ( V ) is then treated with dilute acid solution to give ( W ). ( W ) is first treated with hot alkaline and then very strongly heated to give ( X ).
Final Part
( J ) is reacted with ( O ) in the presence of iron as a catalyst and then treating the product with at very high temperature yields ( Y ).
Finally 2 equivalents of ( Y ) are reacted with one equivalent of ( X ) in the presence of acid catalyst to yield ( Z ).
Find the color of ( Z ) in dilute aqueous solution of ( C ).
Hints.
1) Metal ( A ) gives brick red flame. It's carbonate is insoluble in water.
2) Cation of metal ( K ) belongs to third group of basic radicals (the group).
3) ( F ) and ( H ) are used in metallurgy.
4) ( X ) is an anhydride.
5) ( B ) is a neutral, polar solvent.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
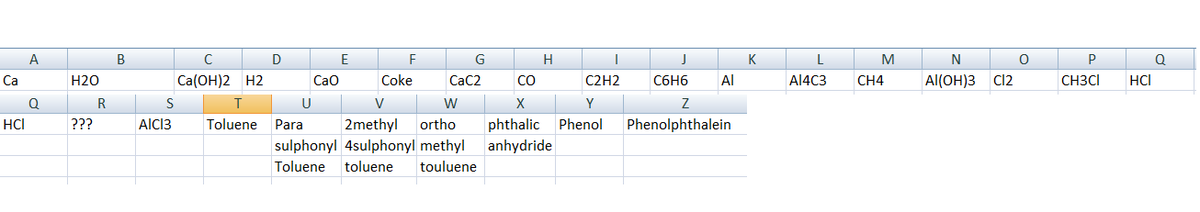
A = C a
B = H 2 O
C = C a ( O H ) 2
D = H 2
E = C a O
F = C o k e
G = C a C 2
H = C O
I = C 2 H 2
J = B e n z e n e
K = A l
L = A l 4 C 3
M = C H 4
N = A l ( O H ) 3
O = C l 2
P = C H 3 C l
Q = H C l
S = A l C l 3
T = T o l u e n e
U=Para-Methyl Benzene Sulphonic Acid
V=3-4 DiMethyl Benzene Sulphonic Acid
W=Ortho-Xylene
X=Pthalic Anhydride
Y=Phenol
Z= Phenolphthalein