Can you boil this water?
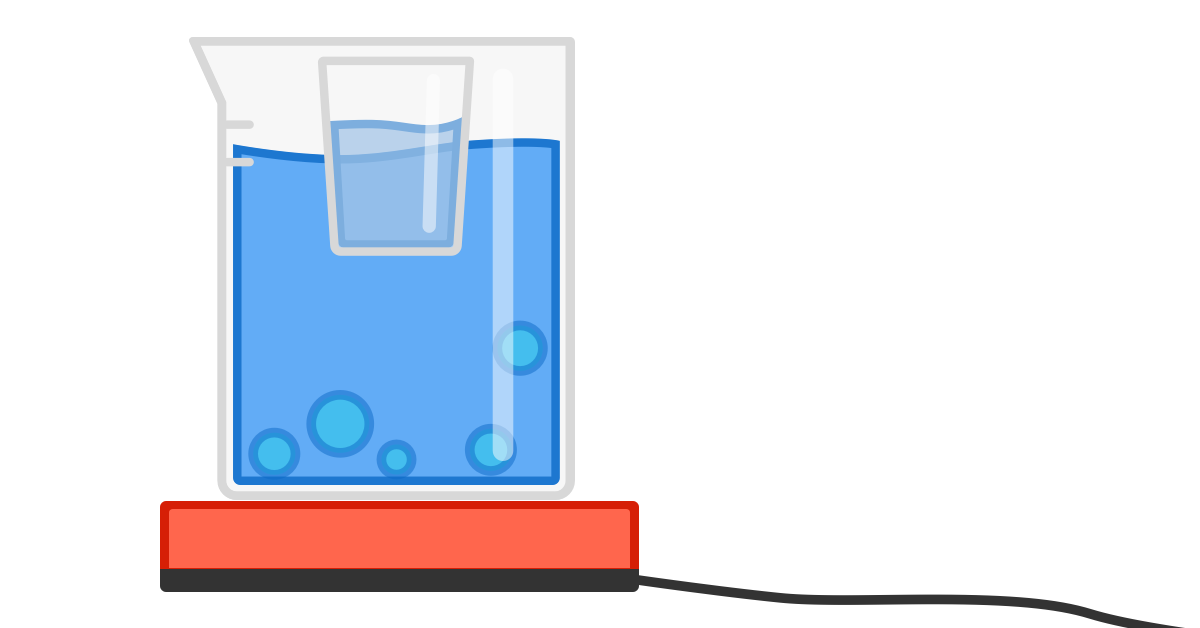
A cup of warm water is suspended in a large pot of water held at a steady boil at atmospheric pressure. Will the water in the cup ever boil?
Assume that the pot never runs out of water and that the environment remains unchanged.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
10 solutions
Discussions for this problem are now closed
I'll bet you could get the water in the cup to boil if the heat source was at 1000 C. In fact, you'd probably get all the water to boil pretty quickly. The containers would probably melt and vaporize too. :)
NORMA NOLASCO but the highest point of boiling of water is only 100 c .sp it is imposible to have the water to boil at 1000 c water would vaporize at 101*c.
At a high enough temperature, the vapor contacting the cup would give the extra heat needed to boil it's contents. You wouldn't need 1000 C. Thank you Norm. This isn't called 'double boiling' for nothing .
You misinterpreted my comment. What if the heat source was the sun? It won't matter where the cup is or what it's made of. The radiant energy would vaporize the whole setup - the water, the container, the cups... all turned into whatever state matter becomes at 5778 K.
@Norm Nolasco – Lucky for us, the water is just at a steady boil.
This is a cuisine method we use in Spain to melt or cook some food but avoid high temperatures. We call it "baño María", that is translated "Maria bath".
Wow, I didn't know about that. I guess you have some way to hang the cup in the water, rather than letting it float in the pot? Thanks for this comment.
Yes, you are wright. I answered this problem wrong because I thought after a while the water was all boiled and the cup did touch the pot. I thought too much...
Technically, the water only boils (becomes water vapor) at the bottom of the pot (bubbles) but these bobbles rise and it appears as if all the water in the pot is boiling. You won't see this bobbing inside the glass for obvious reasons (the bobbles cannot get across the walls of the glass!). So, in conclusion, the water doesn't boil in the glass or in the pot, except for a very small layer at the bottom of the pot which is in contact with the higher than 100 degrees temperature and perhaps the water in contact with some bubbles which could be at higher that 100 degrees celcius (water vapor is 100+ degrees) which depends on how much hotter the stove is.
The question didn't specify whether the cup water system is isolated. In your experience, the system isn't isolated. The heat is loss at the same time it is heated up.
If the water can remain a liquid, then surely the system cannot be in a vacuum due to the pressure needed to maintain it as a liquid.
Yeah, but what I meant was a simplified situation for the practical application sake like those problems in physics textbook. If we have to consider to all those little factors, we will have a whole lot complicated equations unnecessarily.
Would that be a good way to poach eggs?
But the steam from the boiling water o can be more than 100℃! Then there will be heat flow between steam and the cup of water.
But you never mentioned that the heat source heated the water to 100 degrees C... What if it was 500 degrees C?
If the water is boiling, it's temperature wouldn't increase as the energy is being used to break bonds within the liquid rather than heat the water. Therefore, even if the heat source is > 100 degrees, as long as the water is still boiling, it will remain at 100 degrees.
Oh. That is... interesting.
Lots of controversy, good problem :)
I think the question is assuming it is a normal situation though, not some weird optimal situation which we infer from wherever.
The only question would be what does the question mean by 'suspend.' If it is actually held in the water but above the pot, then it wouldn't ever vaporize even if all the water in the pot vaporized. If left free floating then it would.
Interesting side note: If the water below weren't boiling and instead were superheated, the water in the cup could boil.
However, superheating water can be difficult because it requires nearly perfect surfaces. If there are any imperfections (read: nucleation sites) the water will not super heat and will instead boil.
If you want to see how dangerous super heating is, just google "microwave superheated water"
Takes an additional 970 btu/lb to convert 100 celsius water to 100 celsius steam
In reply to Vaidyanathan, I beg to inform him that the question put is whether the warm water in the cup would boil in the given condition. The immediate and simple answer is NO. Because the resultant temperature of the entire quantity of water falls below the boiling point of water as some quantity of heat gets transferred to the water in the cup. Further, as regards, latent heat I would say that it is possible to have water at 100 degree C, simply boiling (liquid state) and So also we can have steam at 100 degree C. Water is available both in liquid and gaseous states at the same temperature of 100 degree C.The factor responsible the the different states of the water at the same temperature is what you have rightly mentioned as Latent Heat. However, reference to latent heat in the present context of the problem on hand was redundant.
I have an interesting thought. Suppose we had a completely insulated column a mile high filled with water. Would the boiling point be the average of the sea level boiling point and the boiling point of water a mile in the air? If so, a cup suspended in the water at the top would boil. Is there a way to figure this out (or what the bp would be)?
So I think there is a way to make this happen though it would be absurd and beyond the scope of this simple example. If you were to take all the bubbles and funnel them to the bottom of the cup of water I can see a scenario where the steam is slightly hotter than the 100C required to boil and the resulting heat transfer from it being concentrated at the bottom of the cup would result in the cup boiling. I guess the real question is does the steam actually achieve temps above 100C or does the contact with the burner end the moment it becomes steam, resulting in 100C water and 100C steam which would not allow the cup to boil.
Then how does a double boiler work?
EXCELLENT EXPLANATION. YOU ENLIGHTENED ME. THANK YOU SIR.
This is a well known cooking technique. In my country it's called Baño María. The water inside the cup doesn't boil because it doesn't reach the necessary amount of energy. Of course, if the water outside of the cup was saltier than the water inside, then the cup would boil.
answer is no... it acts like a water bath... the cup water reaches 100 centigrade and transfer of heat is stopped as the outside temperature of boiling water is also 100. further phase change of cup water to steam needs latent heat which cant happen as heat transfer is stopped. provided the whole system is under std atmospheric pressure. close example is household milk cooker
answer is no why? as when both the containers will have same temperature =100degree means the water in upper container will start boiling .
Water needs additional heat to boil, even at 100 degrees it still needs more energy to boil.
But the outside water is boiling? So the inside could also boil?
From an engineering point of view:
Heat is needed to increase temperature and change the phase of water in the cup.
Heat transfer can only happen if there is temperature difference.
The reason the water in the pot can boil is because there is temperature difference between the water and the heat source. Since there is also temperature difference between the water in pot and cup, heat transfer into cup happens to increase the temperature of the water in cup to its saturation temperature. However since the water in the pot is always boiling, its temperature is constant so when the water in the cup reached the saturation temperature as saturated liquid, there is no temperature difference between water in cup and pot so no heat can be transferred into cup to change its phase to gas.
I think what some are missing here is that the only water that is actually "boiling," or changing state to vapor, is the water in contact with the pot at the heat source, the bottom. Although it appears the entire pot is boiling, depending on your definition of boiling, it is not. The only reason the water is boiling is it is at 100 degrees and then receives more energy to push it to vapor. The suspended cup being no where near the heat source and not receiving any further energy will not boil.
This is a "double boiler", I've used it for cooking/baking with chocolate. The practical purpose is that the cup (and it's contents) never get hotter than boiling water, so the chocolate will not burn.
I've also seen this in my thermodynamics courses. The liquid water in the pot will never get hotter than boiling, so the liquid in the cup can reach equilibrium with the liquid in the pot, but not get any hotter.
It is possible that vapor bubbles (> boiling temp) will contact the cup, transferring some energy, facilitating the transition from liquid to vapor inside the cup. However, this would require a very hot source in the pot to produce enough vapor bubbles to heat the cup above boiling.
My answer to " Will the water in the cup ever boil?" is: Probably not; it depends on the temperature/heat transfer from the heater to the liquid in the pot.
To change phase from a liquid to a gas, water must first reach it's boiling point and then additional energy must be added for it to change to a gas. That is. liquid water at 100C has significantly less heat than steam at 100C. Since the pot of water is at boiling temperature any additional heat applied goes toward creating steam, not raising the temperature of the water. Because heat flows due to a temperature difference, the water in the cup will not receive any additional heat once it reaches equilibrium with the water in the pot, therefore it cannot boil.
Boiling is basically phase transfer of the liquid. Heat is absorbed to make the phase transfer. Now the heat required to maintain the boiling keep the water at 100 deg C. And the cup is also maintained at 100 deg C. However it does not receive the extra heat to convert it into steam, i.e. Latent Heat!!!
We all know that water boils at 100°centigrade,it does not get more energy to turn into steam,hence it will not boil
The water will remain at a temp of 100 degrees but will never heat the Styrofoam enough to cause the water inside to boil. At the heat of vaporization, liquid water can not get any hotter, thus undergoing a phase change to gas, the cup's specific heat capacity will prevent the water from ever boiling inside of it.
Water at 100 degrees Celsius needs more heat to boil. That is, water will boil at room conditions if and only if heat is added to it after its temperature is 100 degrees Celsius. This is the energy required for liquid water to convert into steam at 100 degrees. This energy is called the latent heat of vaporization, as it is "hidden". This is also the reason why the temperature of water remains constant throughout boiling. The heat is used to turn water into steam and not to raise its temperature.
We know that the heat flow between two bodies will stop once they are at the same temperature. So what happens here is that the water outside the cup and in the cup both get to 100 degrees Celsius. The water outside the cup receives more heat from the heater and starts boiling(keep in mind that the temperature is always 100 degrees throughout boiling). But as the cup and its surroundings are in equilibrium (same temperature), there is no heat being transferred into the cup. This means that the water in the cup is at 100 degrees, but it doesn't get more energy to turn into steam, and hence it does not boil.
Edit:
Guess what? I tried doing this experiment at home and it came out with favorable observations, even after 10 mins of continuous boiling, the water in the floating cup did not boil! Here is the pic(tried adjusting brightness and contrast to bring out difference):
Also, I uploaded a video of this on youtube, here's the link.