Cool It!
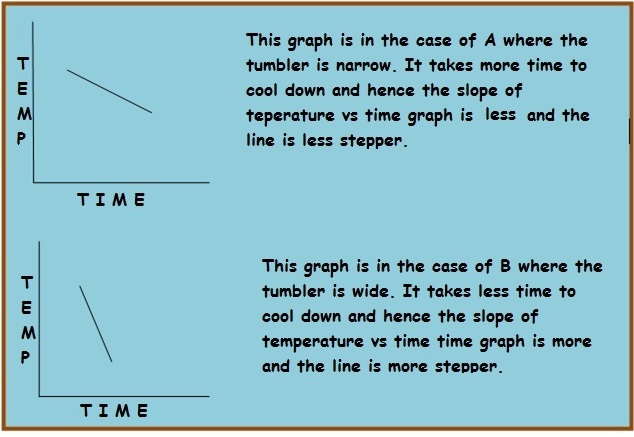
A
is narrow whereas
B
is wide.
You are provided with two tumblers A and B , one narrow and the other wide, as shown in the diagram. Both contain the same amount of hot water whose initial temperature is the same.
Now you keep them open on a sunny day under similar conditions.
In which tumbler do you expect the water to cool down faster ?
Assume that the tumblers do not absorb heat and are insulated.
Try my World of Physics to solve many problems like this one.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
14 solutions
Wait! Why is the rate of cooling down got any relationship with rate of evaporation?
Log in to reply
Gases are at greater energy than the liquid state. Because of forces of attraction between the liquid molecules. Hence, some energy must be given to the liquid so that evaporation takes place. This energy is supplied by the liquid itself in the form of heat. Hence, the cooling.
Si Cheng, evaporation produces a cooling effect!
Why is there no discussion whatsoever about heat conduction through the sides of the cilinder? Tumbler A has much more surface area exposed and can lose heat through radiation and conduction to the surrounding air easily
Log in to reply
Exactly. Unless the material of the Tumblr is a perfect insulator, I think that should be better explained on the problem
Tumble b has the larger surface area.... 2 pi r square + 2 pi r height...for the same volume the shorter wider tumble has more surface area on both open end and walls and bottom
The Tumblr is made by insulator
This is true. I'd just add that conductive heat transfer between the liquid and the atmosphere is possible even if there is no evaporation. So even without evaporation, B cools faster than A.
Are we ignoring the cooling effect from radiation?
I think it depends on the relationship of h and r
Yes, as the tumblers are insulated, we ignore radiation.
We know that the
rate of evaporation and cooling of water is directly proportional to the surface area on which it is kept
. That means more the surface area of the container in which water is kept more is the rate of evaporation or cooling of water. Therefore, water kept in tumbler
B
will cool down faster as it has more surface area.
What if I narrow A to such an extent that it becomes a capillary?
Log in to reply
Then at one point no cooling takes place.
Log in to reply
Umm... I don't really get that. What I mean to say is suppose I have tumblers A and B and A is almost a capillary but B is a wide cylinder (just like the one in your picture). I fill both of them with water and place them in a hot room. After some time the water inside both of them will have the same temperature due to attaining thermal equilibrium. I take both the tumblers out of the hot room (at the same time) and place them in a cool place (at the same time). In that case what I think is that the water in A should cool down faster than B.
Log in to reply
@Abha Vishwakarma – Ooh.. I forgot the assumption that the tumblers won't absorb heat. I get it now.
But another question comes into my mind that what if they did absorb heat? If I narrow A to such an extent that it becomes a capillary and widen B to such an extent that it becomes equivalent to a simple plate, then in that case will water cool down at the same time in both of them?
Log in to reply
@Abha Vishwakarma – May be it may happen. Or there can be a slight difference between their time to cool down.
*directly proportional
If the tumbler were to also absorb heat does the result change?
It can be argued that A will cool faster by radiating heat, since it has a higher total surface area than B. You should specify that the tumbler is insulated if you want the answer to be B.
Log in to reply
I am also not sure about it but my mother says B, try this experiment at home, so that you may get clarity, boil milk and use put some milk in glass and bowl and observe which gets cool first and let me know in first place
Cooling is also achieved via air convection against the side walls, you should stipulate that those are insulated otherwise, theoretically, tumbler A could cool down faster.
Log in to reply
But it is mentioned that the tumblers do not absorb heat.
What if it's a sunny day in the rain forest? The relative humidity is 100% so net evaporation is zero. The tumblers could be actually heating up by condensation and convection if they are cooler than the ambient environment.
B has more exposure to the surrounding due to more surface area which results for faster cooling than A.
Rate of evaporation increases with an increase in surface area. So, B will have greater rate of evaporation, thus more heat loss.
B has more surface area for cooling
The rate of evaporation of a fluid such as the liquid concerned is proportional to the area of the surface exposed to tho atmosphere. Also note that the temperature difference between the surroundings of the glass and the glass itself play a role in determining the rate of cooling
Emission flux is same for both containers but since surface area of B is higher it radiates more energy at a given time: Radiated power = flux*Area.
Heat dissipation is directly proportional to the surface area for any body..so B is the right answer
Deeper water is always colder.
The rate of heat loss is directly proportional to surface area. You figure out the rest 😉 i believe in you!
A is also possible. if A is very very long.Then?
The first jar will cool slower because it has a smaller surface area.
I wasn't familiar with a quantitative expression for this so I just reasoned from an extreme case: a very large and very shallow body of hot water will trade off its heat to its environment much more quickly than a very narrow and very deep reservoir of the same water, since more of the water molecules can impart their momenta to molecules in the air in the former case than in the latter. Deep in the reservoir, the average kinetic energy of a water molecule is very high (and thus it's surrounded by other molecules of high average kinetic energy) and so the molecules will trade energy with one another but not with any regions of lower average kinetic energy. Only at the surface can the average kinetic energy of a region of molecules be reduced, so more surface means quicker cooling.
Heat current=AK(∆t)/d. (Well known)
Given, A1.d1=A2.d2 (A1<A2)
We get Q1/Q2=(d2/d1)^2<1
So, Q1<Q2
rate of evaporation is proportional to surface area of the container. Clearly, B has more surface area. So the water will cool down faster in tumbler B