I hate Mosely
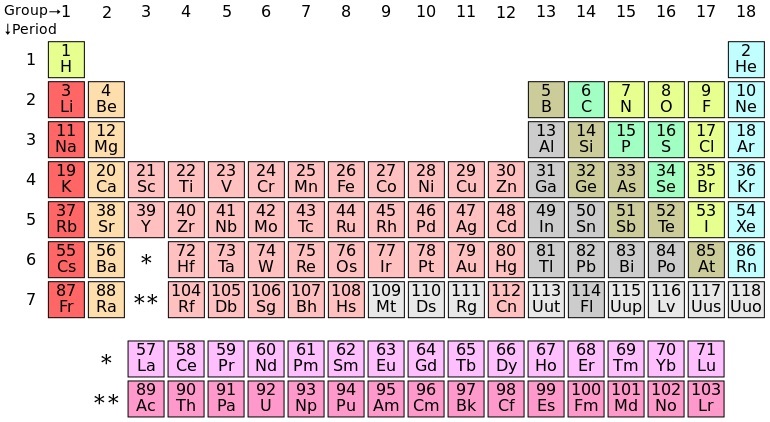
The m o d e r n p e r i o d i c t a b l e given by mosely as shown above,
Which of the following element is not a transition element ?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
1 solution
It's the IUPAC defnition that you've used.
Most scientists describe a "transition metal" as any element in the d-block of the periodic table, which includes groups 3 to 12 on the periodic table.
It all depends on defnition
Log in to reply
Well then all the options lie in Group 3 to 12 . Zinc does not fall under the category of transition metals because when it has a completely filled d orbital.
Log in to reply
Log in to reply
@Kishore S. Shenoy – Even though Zn is a part of d block.Its not counted as a transition metal (Thanks to the IUPAC definition of transition metals.Check this ).The question was to find the element which is not a transition metal btw.
Log in to reply
@Athiyaman Nallathambi – So, it should've been mentioned "according to IUPAC defnitions". Otherwise no answer is true.
Log in to reply
@Kishore S. Shenoy – The question was to Find the element which is not a transition metal.Obviously all of these elements are a part of d -block.Like @Akhil Bansal said, most people think that all d block elements are transition metals.This is not true in the case of Zn , Hg , Sc and Cd.
But given the periodic table we could have just checked whether the options lie between the groups 3 and 12.
Log in to reply
all of them lie in between 3 and 12
Log in to reply
That's why i added this question,because many people thinks that all
d-block
elements are
transition elements
,which is not true.
Z
n
,
H
g
,
S
c
and
C
d
belongs to d-block but aren't transition elements.
Right.Its my mistake.Both Scandium and Zinc are not counted as transition elements because Zn ion has completely filled d orbital and Scandium when it forms ions has no d electrons.Thanks for pointing out my mistake.
Definition: Transition element is an element whose atom has a partially filled d sub-shell, or which can give rise to cations with an incomplete d sub-shell
However, Zinc has fully filled d-orbital and thus cannot be a transition metal.Its atomic number of Zinc is 30, electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 (Filled d -orbital).