Polarity of molecule
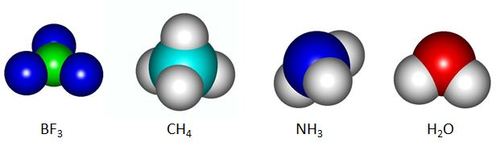 In chemistry, a space-filling model, also known as a calotte model, is a type of three-dimensional molecular model where the atoms are represented by spheres whose radii are proportional to the radii of the atoms and whose center-to-center distances are proportional to the distances between the atomic nuclei, all in the same scale. The above are space-filling models of four molecules. In a liquid state, which of these four substances will be attracted by an electrified body?
In chemistry, a space-filling model, also known as a calotte model, is a type of three-dimensional molecular model where the atoms are represented by spheres whose radii are proportional to the radii of the atoms and whose center-to-center distances are proportional to the distances between the atomic nuclei, all in the same scale. The above are space-filling models of four molecules. In a liquid state, which of these four substances will be attracted by an electrified body?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Among the 4 molecules, N H 3 and H 2 O are the only molecules that have an appreciable dipole moment (due to difference in electronegativity).