Propane: A Metabolite Precursor
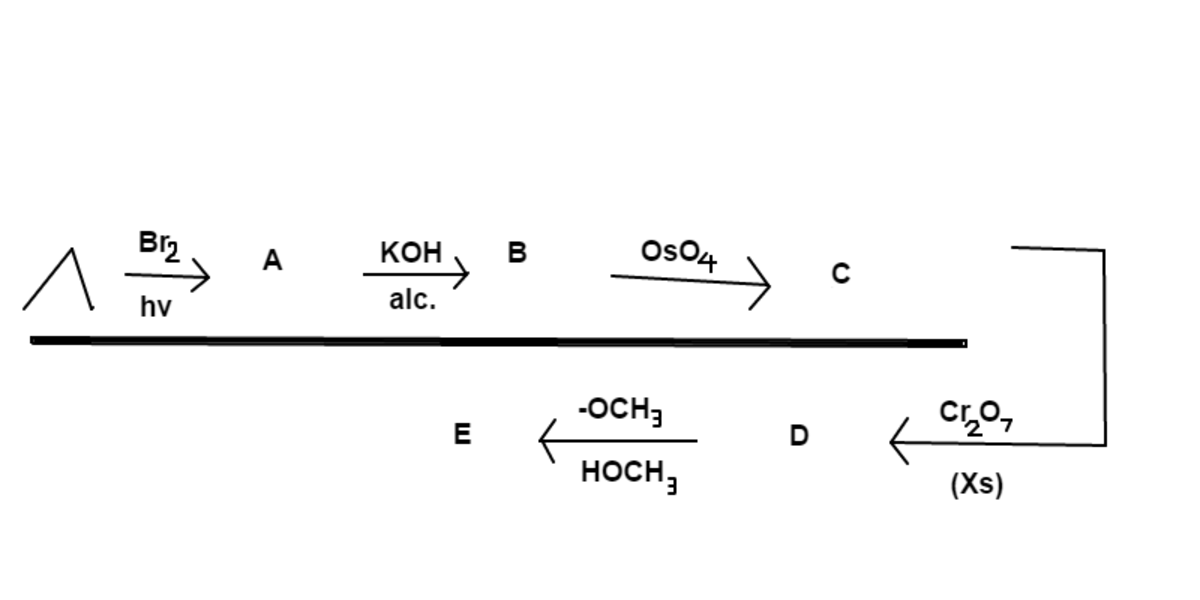
The reactions above start with propane . Find the number of atoms in the final product .
Notes:
- hv indicates light
- alc. indicates alcohol
- Xs indicates excess
David's Organic Chemistry Set
David's Physical Chemistry Set
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
P r o d u c t A : Step 1) Bromine atom is added to 2 ∘ carbon P r o d u c t B : Step 2) Removal of halogen forms a double bond P r o d u c t C : Step 3) Osmium Tetraoxide acts on the alkene to form a diol P r o d u c t D : Step 4) Dichromate acts as a powerful oxidant that oxidized the 2 ∘ alcohol to a ketone and the 1 ∘ alcohol to a carboxylic acid to form the metabolite pyruvate P r o d u c t E : Step 5) The carboxylic acid becomes a methyl ester through the process of transesterification The final product E is methyl pyruvate ( C 4 O 3 H 6 ), a.k.a methyl-2-oxopropanoate.
A n s w e r : 4 C + 3 O + 6 H = 1 3 a t o m s