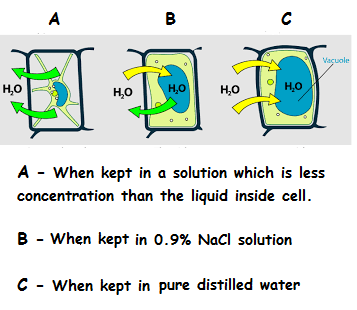
Salt solution vs Distilled Water
A biologist conducting experiments with animal cells wants to observe them under a microscope. Animal cells are semi-permeable, meaning that molecules like water and oxygen can move freely in and out of the cell, but charged ions cannot.
She prepares the slides by placing the cells in a 0.9% N a C l solution (to form a seal between the slide and the base).
What would happen if she used distilled water instead of the salt solution?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
6 solutions
The experimenter is a woman!
Log in to reply
Ya the experimenter is a woman I agree with you
The legend of your figure mixes up case B (equal flow in and out of the cells), "When kept in normal pure water" (in distilled water makes more sense) and C (only inflow of water), "When kept in 0.9% NaCl solution.", countering the whole point you try to make in the text above... Can you please fix the figure?
Log in to reply
So true. I would've commented about this myself if noone had mentioned this screw-up...
If the cells could of been from only one place in a specific organism then the answer could have a different one
It is not stated that the cells are in equilibrium in the 0.9% NaCl solution, or that the (female) scientist was successful in conducting their experiment. I'd prefer the answer to be that the "cells enlarged" to "the cells burst", as I see nothing in the question that means that it is guaranteed that the cells take on so much water that they burst.
Log in to reply
The question will not mention anything about equilibrium of cells rather it indirectly gives us the clue by telling that the cell is in normal condition when placed in 0.9 NaCl solution. From this we can conclude that the concentration of this cell is 0.9
Log in to reply
Not even that. It only says that "she prepared ... With 0.9"... It does not say it was "successful" or in "normal condition"
They aren't talking about animal cells. Animal cells don't have a vacuole or are shaped like a rectangle
Log in to reply
sorry! plant
We are taking in general as "cells" whether animal or plant cells as osmosis occurs at both the cells.
Ummm humans are animals to,aren’t they?
Information in originsl problem insufficient. Diagrams do not correlate to text below
Log in to reply
Ok. Then can you please tell me where is the problem in the diagram and text.
Log in to reply
The diagram shows B as equilibrium (some water going in and a similar amount going out), but the caption says B is normal pure water, and shows C as a hydrophilic process that would probably end up with a burst cell... while the text says C is the 0.9% solution. Clearly there's a mismatch there.
Log in to reply
@C . – I have fixed it. Now you can check the diagram
Log in to reply
@Ram Mohith – That's great... Except i think i forgot to mention the part where A is incorrect too: it needs to be when the cell is kept in a solution of HIGHER concentration, that's when the water will seep out to dilute the environment. :-B
Well they mean any living thing and any part so it doesn’t matter what area the cells are from
"Basic" this promblem should be in "Intermediate" or "Advanced".
Log in to reply
Not necessarily "Advanced" but can be placed in "Intermediate".
the experimenter is a woman!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Log in to reply
No he's not. :-p
I just mentioned as "biologist".
I agree there is not enough information is the original question to solve this. Most people that were able to solve this have some knowledge about cellular biology.
Totally not math
Just came here for the math
Water moves in and out of the animal cell through the semi-permeable membrane through a process called osmosis . Osmosis is the spontaneous net movement of solvent molecules (in this case, water) through a selectively permeable membrane into a region of higher solute concentration, in the direction that tends to equalize the solute concentrations on the two sides.
If distilled water is used, which has zero solute concentration, there would be a continuous net movement of water into the cells and the cells would burst . A 0.9% saline (NaCl) solution is used to provide the osmotic pressure or the solute concentration necessary to prevent the excessive movement of water into the cells.
By osmosis, water from outside the cells would move into the cells, causing them to burst.
Osmosis moves from most pure to least pure. Due to the distilled water having no other molecules in it other than water, thus the water goes into the cell causeing it to inflate then burst after a certain amount of time.
This is an osmosis question. The entire concept revolves around the concentration of solutes dissolved in solutions. Water molecules will move from an area of high concentration to an area of low concentration. Water can pass, unaided(passively), through a semi-permeable membrane, as is an animal cell membrane. Solutes, are dissolved particles, usually, in living animals, composed of salts, minerals, proteins and sugars, atoms and molecules. Without dissolved solutes the water solution could be 100% composed of water molecules. Compare this to a solution with some solutes dissolved in it. Solutes have mass and occupy space. The more solutes dissolved between the water molecules, the more space the solutes occupy and the less space the water molecules occupy. If you place a semi-permeable membrane between both solutions the area of low concentration of water is the one with the higher concentration of dissolved solutes. The area with the highest concentration of water is the solution with no solutes dissolved within it. So, if water will naturally desire to move from an area of high concentration to an area of low concentration, which direction will it flow across the semipermeable membrane? The pure water is on the left side of the membrane and the dissolved solutes solution is on the right side of the membrane? Remember, the solutes cannot passively move across the semipermeable membrane, only the water can. Correct, left to right!!🙋
Simply, osmotic pressure. Water goes through highly concentrated place. In NaCl, Concentration is quiet similar. But distilled water, however, water moves into cell, stir up the burst of it.
Using 0.9 % NaCl Solution :
The biologist was successful in conducting her experiment. That means the concentration in out of the cell are equal so there is no net movement of water and the cells are in their usual size. so, we can conclude that the concentration inside cell is 0.9 .
Using Distilled Water :
Distilled water is neutral. So, it concentration is 0. Now, if the same cell is kept in distilled water it means, the concentration in the surroundings of the cell is less than that in cell . So, water moves into the cell from surroundings due to osmosis . Therefore, after some time as more water will come inside the cell and enlarges it to some extent after which it bursts.
The below picture tells about the condition of cell when kept in different solutions.