The Dark Carbon Rises
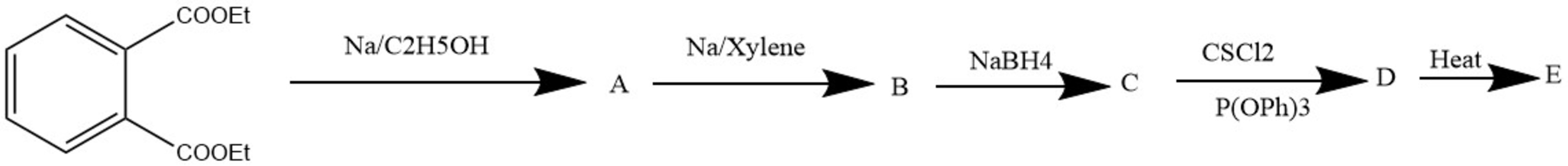 Compound E reacts with atomic Carbon to give F. Knowing that F is a hydrocarbon with
and that after deprotonation, the anion is aromatic and has only 4 signals in H-RMN, find the number of signals for F in H-RMN.
Compound E reacts with atomic Carbon to give F. Knowing that F is a hydrocarbon with
and that after deprotonation, the anion is aromatic and has only 4 signals in H-RMN, find the number of signals for F in H-RMN.
Hint: C is a diol and E has 4 double bonds.
The answer is 7.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The sequence of reactions is as follows : Birch reduction ( the reduced double bond is the one between the carboxylic groups), Acyloin condensation, reduction of the carbonil group, Corey reduction of the diol which leaves a double bond so D is bicyclo[4,2,0]octa-2,4,7-triene; ring-opening to COT and reaction with atomic carbon followed by a rearrangement to indene.